I am Professor Mahan, I received my Ph.D. in Genetics from the University of Utah. I was an NIH post-doctoral fellow at Harvard Medical School where I began my work on the molecular mechanisms underlying Salmonella pathogenesis. I joined the UCSB faculty in 1993 and was co-founder and Director of Remedyne Corporation, a biotech company in Santa Barbara, California.
My lab investigates the molecular mechanisms underlying microbial pathogenesis and the associated innate and adaptive immune responses that increase disease and compromise host immunity. The applied goal is to translate this research into effective therapeutic strategies for the diagnosis and treatment of multidrug-resistant pathogens and microbial sepsis.
Half of the world’s population has a smartphone. We wanted to bring low-cost, state-of-the-art diagnostics to resource-limited settings.
Currently, PCR testing is the gold standard of COVID-19 diagnosis. However, like all methodologies, it has its limitations. What are the limitations of using PCR for COVID-19 diagnosis, and how have these limitations affected the course of the COVID-19 pandemic?
PCR is the gold standard, but it is slow (results in days) and very expensive ($100-150/test). Further, PCR requires expensive equipment and trained personnel, which is why samples are sent to a designated lab facility rather than a home-based test.
Another COVID-19 detection method is the Rapid Antigen Test. These tests are fast and low cost ($10-12/test) but are not very accurate. If you are positive, you are positive, but if you are negative, you may not be negative. Because of the high false-negative rate, antigen testing before gatherings has led to a lot of infections.
Your latest work has transformed a simple smartphone into a COVID-19 detection kit. Can you describe how you developed the smaRT-LAMP app and the methodology behind it?
The app and lab kit uses a smartphone camera to measure a chemical reaction and determine a diagnosis in 25 minutes. The chemical reaction contains a fluorescent dye that glows when the virus is amplified- more glowing, more virus. Each test costs less than $7 vs $100 to $150 per PCR test.
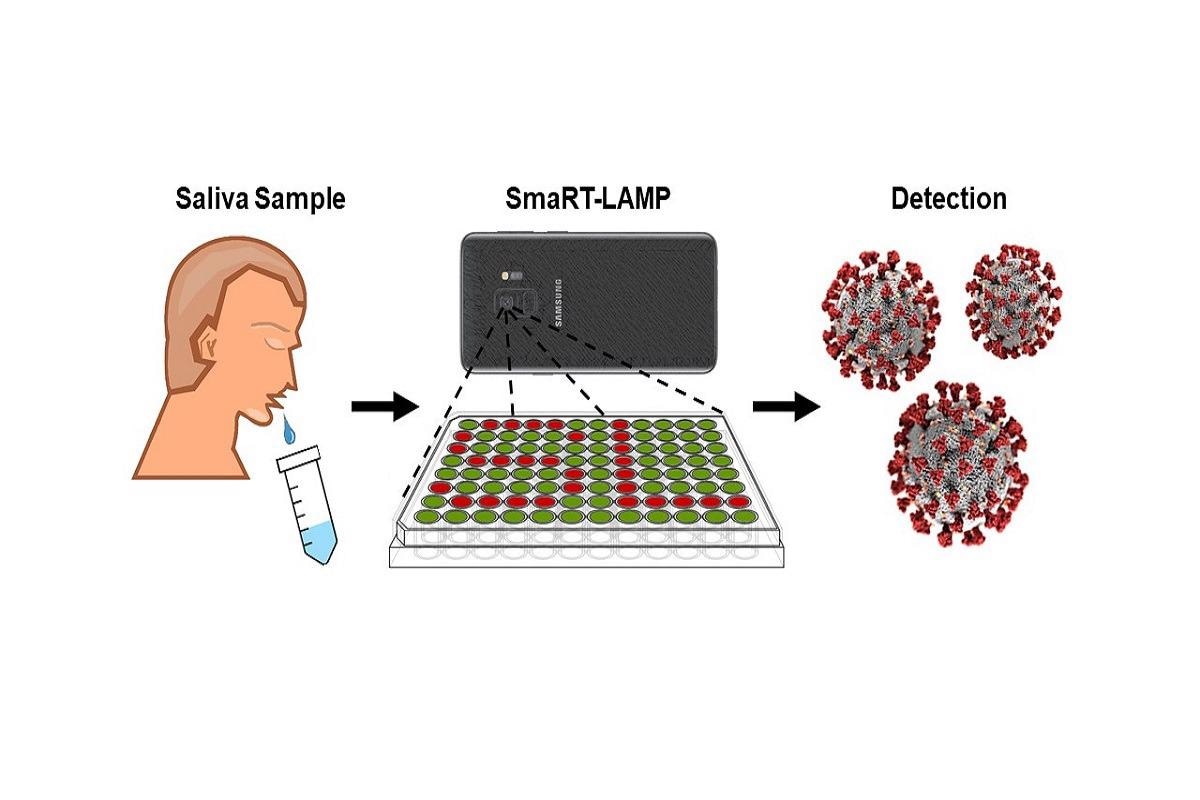
You highlight that smaRT-LAMP can be used worldwide for a relatively low price. Considering global economic and health disparities, how may this technology transform COVID-19 testing in developing countries?
The low price of the technology and test will enable widespread testing in developing countries. We have provided the app and technology open source and freely available to all. The Bacticount app is available on Google Play; the reaction mix and recipe are available from the manuscript.
The device was fabricated for <$100, and each test costs <$7. 90% of the test cost is enzymes. If the test is done in large quantities, we believe it can get down to $2/test for COVID and Flu. We have previously shown that urinary tract infections can be done for $1/test.
smaRT-LAMP can differentiate between SARS-CoV-2 and influenza. Given the similar respiratory symptoms of both infections, how may this transform the current issue of misdiagnosis?
The lifting of pandemic restrictions exacerbates the potential for a dual epidemic of COVID-19 and influenza, termed a “perfect storm”, due to a potential increase in severe illness, transmission, and misdiagnosis resulting from symptom overlap. SmaRT-LAMP can prevent misdiagnosis by identifying the infecting pathogen(s).
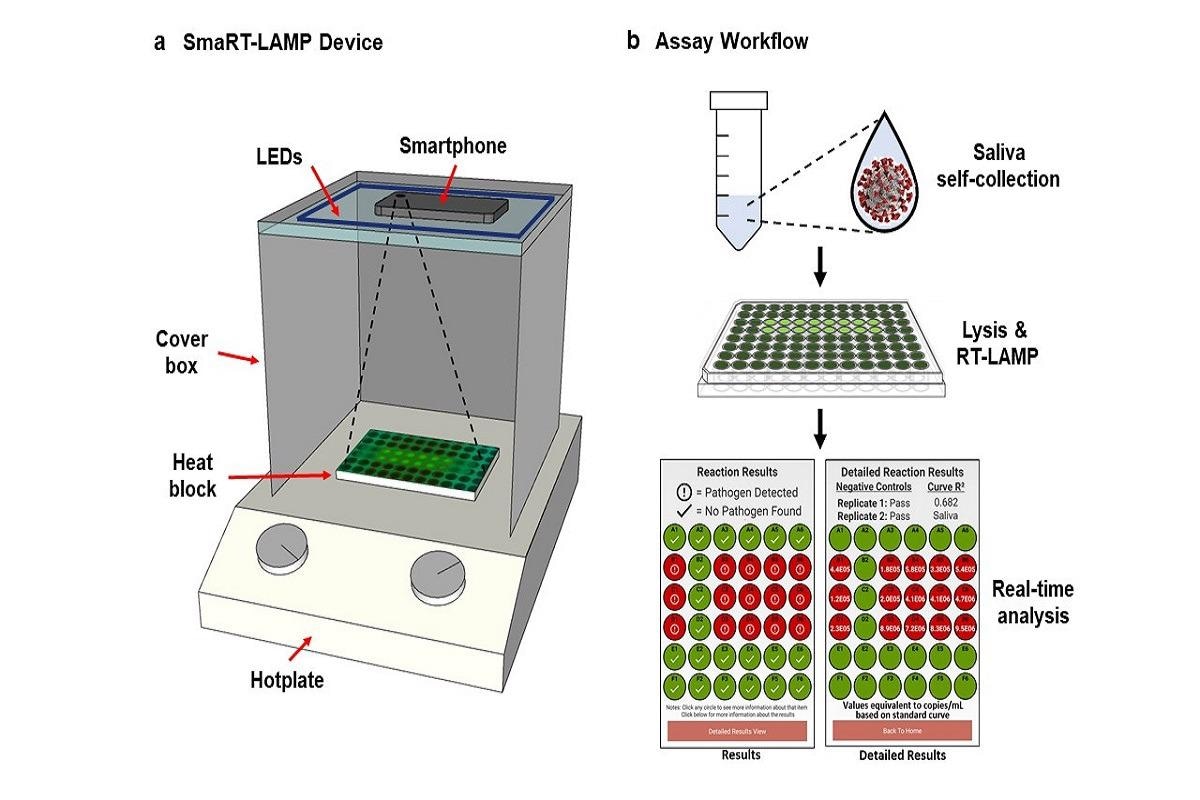
Given growing concerns of emerging infectious diseases and future pandemics, could the smaRT-LAMP system be adapted in the future for novel viruses?
The test can be readily adapted for other pathogens with pandemic potential including novel COVID variants and influenza. SmaRT-LAMP has been shown to detect many pathogens, including Staphylococcus, Streptococcus, Salmonella, E. coli infections; and it works on human blood, urine, feces, and saliva.
During the course of the pandemic, smartphone technology has had an important role to play, for example, track and trace apps and now this detection kit. How do you see smartphone technology developing as we live with COVID-19 and potentially face another pandemic in the future?
Telemedicine will be critical in the next phase of the pandemic and in future pandemics. It is much cheaper than testing in a healthcare setting, with the added benefit of reduced exposure to the patient and healthcare personnel.
This work involved collaboration between different scientists. How important was this collaboration to the outcome of your work?
The team consisted of academic scientists with expertise in microbiology, genetics, and biochemistry, along with infectious disease physicians at our local hospital that were instrumental in the study design as well as patient sample collection.
What’s next for you and your research?
The next step is to expand the compatibility of the app to the latest Android and Mac operating systems. The study was designed to meet FDA standards, and we are considering applying for a EUA (Emergency Use Authorization).
Where can readers find more information?
About Michael J. Mahan
Dr. Mahan was recruited to the University of California, Santa Barbara in 1993, and holds appointments in the Molecular, Cellular and Developmental Biology, and Biomolecular Sciences and Engineering programs.  Recognitions include AAAS Newcomb-Cleveland Prize, Top Paper of the Year, Science, 1994; American Cancer Society Junior Faculty Research Award, 1995; Beckman Young Investigator Award, 1995; Harold J. Plous Young Faculty Award, 1998; Editorial Board, J. Molecular Medicine, 2001–2004; Faculty Distinguished Teaching Award, 2006; USDA National Impact Paper, 2008; EbioMedicine, Top Papers of the Year, 2015.
Recognitions include AAAS Newcomb-Cleveland Prize, Top Paper of the Year, Science, 1994; American Cancer Society Junior Faculty Research Award, 1995; Beckman Young Investigator Award, 1995; Harold J. Plous Young Faculty Award, 1998; Editorial Board, J. Molecular Medicine, 2001–2004; Faculty Distinguished Teaching Award, 2006; USDA National Impact Paper, 2008; EbioMedicine, Top Papers of the Year, 2015.
Dr. Mahan was Founder and Director of Remedyne Inc., a biotech company involved in vaccine development, 1999–2003. Modified live attenuated Salmonella vaccines are currently undergoing clinical trials for use in commercial livestock production.