The majority of SARS-CoV-2-infected people do not require hospitalization. Nonetheless, a small percentage of individuals develop long-term consequences after SARS-CoV-2 infection, known as long COVID. Fatigue is a frequently reported ailment among the COVID 2019 (COVID-19) convalescent individuals and has a significant negative influence on the quality of daily life. Further, fatigue seems to be a multi-system disorder, including hormonal, metabolic, and immunological abnormalities. However, the neural mechanisms associated with post-COVID fatigue are not yet understood.
About the study
In the present study, the scientists conducted a series of non-invasive behavioral and neurophysiological assessments evaluating the autonomic, peripheral, and central nervous systems in subjects with self-reported fatigue following a mild form of COVID-19 to characterize the neural mechanisms underlying post-COVID fatigue.
A total of 37 participants with no history of hospitalization during the mild SARS-CoV-2 infection underwent an approximately four-hour laboratory investigation around six- to 26-weeks after SARS-CoV-2 infection. Further, 52 age- and gender-matched individuals served as controls for the study.
The battery of well-characterized behavioral and neurophysiological tests yielded 35 measurements. Of the 35 measurements, 33 depicted the condition of various nervous system components, and the remaining two were tympanic temperature and blood oxygen saturation.
Findings
The results reveal that a history of mild COVID-19 was associated with reductions in activities of particular cortical circuits, dysregulation of myopathic change in skeletal muscles, and autonomic function relative to gender- and age-matched cohorts without fatigue.
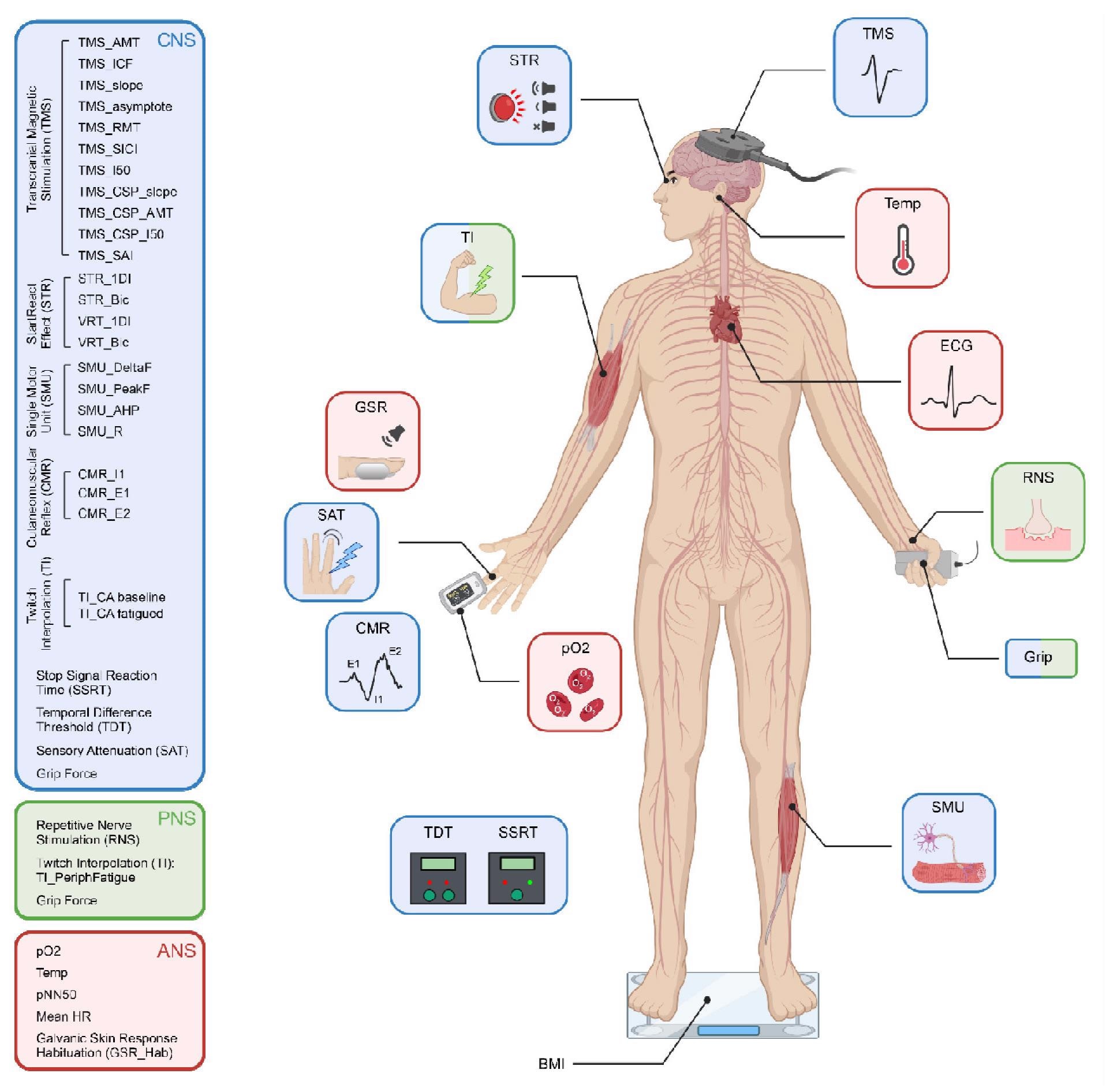 Schematic representation of the different tests performed, colour coded according to which components of the central, peripheral and autonomic nervous systems (CNS, PNS, ANS) they assessed. TMS, transcranial magnetic stimulation; ECG, electrocardiogram; RNS, repetitive nerve stimulation; SMU, single motor unit recording; BMI, body mass index; TDT, temporal difference threshold; SSRT, stop signal reaction time; pO2, blood oxygen saturation; CMR, cutaneomuscular reflex; SAT, sensory attenuation with movement; GSR, habituation of the galvanic skin response to loud sound; TI, twitch interpolation, STR, StartReact effect.
Schematic representation of the different tests performed, colour coded according to which components of the central, peripheral and autonomic nervous systems (CNS, PNS, ANS) they assessed. TMS, transcranial magnetic stimulation; ECG, electrocardiogram; RNS, repetitive nerve stimulation; SMU, single motor unit recording; BMI, body mass index; TDT, temporal difference threshold; SSRT, stop signal reaction time; pO2, blood oxygen saturation; CMR, cutaneomuscular reflex; SAT, sensory attenuation with movement; GSR, habituation of the galvanic skin response to loud sound; TI, twitch interpolation, STR, StartReact effect.
In detail, the maximal grip strength was not substantially declined in pCF, indicating that the force generation for short contractions was intact in these cohorts. Further, the intrinsic motoneuron excitability and the efficacy of transmission at the neuromuscular junction evaluated using various measures suggested that these parameters were comparable between the control and the study groups. Nevertheless, pCF patients were associated with a high level of peripheral fatigue than the control group while evaluating alterations during extended maximal contraction, suggesting pCF causes metabolic changes in muscle fibers resulting in lowered force generation following prolonged activity.
Intracortical facilitation (ICF) was substantially lower in pCF subjects relative to the control groups while evaluating the functioning of the primary motor cortex. However, several measures of cortical inhibition did not show any notable dissimilarities among the study and control lots. In addition, various assessments of sensory function demonstrated no substantial variations between the pCF and control groups, indicating that disruptions in sensory feedback might not be a contributory component in pCF.
The pCF subjects exhibited a higher resting heart rate than the age- and gender-matched cohorts. Further, variations were also seen in other assessments of autonomic functions such as tympanic temperature, galvanic skin response habituation, and heart rate variability among the study and control groups. These findings are indicators of elevated vagal tone compared to sympathetic, implying that pCF individuals were associated with a degree of dysautonomia.
There were differences in a minimum of three neurophysiological measurements between the pCF subjects and control lots. In addition, a marked variation in blood oxygen saturation was also seen between the study and control lots. Nonetheless, cluster analysis did not demonstrate any subgrouping, indicating post-COVID fatigue was a single entity with inter-personal variation instead of a few discrete syndromes.
Conclusions
The study findings connotate the urgency for a better understanding of the physiological underpinnings of long COVID, particularly pCF. It is crucial to know which neural systems are affected in pCF to design effective treatments. Thus, the aberrations in behavioral and neurophysiological tests observed in the current study among the pCF individuals and controls might point to new directions for principled therapeutic intervention in the pCF treatment.
Further, if these abnormalities appear early following SARS-CoV-2 infection, they could serve as quick and accurate biomarkers for diagnosing and tracking the development of fatigue with time.
Collectively, the study shows that dysregulation exists in three major divisions of the nervous system during pCF by utilizing tests that are simple to perform and can be readily integrated into future studies to assess and devise treatment strategies for pCF.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Neural Dysregulation in Post-Covid Fatigue, Anne ME Baker, Natalie J Maffitt, Alessandro Del Vecchio, Katherine M McKeating, Mark R Baker, Stuart N Baker, Demetris S Soteropoulos, medRxiv 2022.02.18.22271040; doi: https://doi.org/10.1101/2022.02.18.22271040, https://www.medrxiv.org/content/10.1101/2022.02.18.22271040v1
- Peer reviewed and published scientific report.
Baker, Anne M E, Natalie J Maffitt, Alessandro Del Vecchio, Katherine M McKeating, Mark R Baker, Stuart N Baker, and Demetris S Soteropoulos. 2023. “Neural Dysregulation in Post-COVID Fatigue.” Brain Communications 5 (3). https://doi.org/10.1093/braincomms/fcad122. https://academic.oup.com/braincomms/article/5/3/fcad122/7115845.