The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by the rapid outbreak of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has massively impacted the global healthcare system and economy. SARS-CoV-2 is an RNA-based respiratory virus that belongs to the family Coronaviridae.
Scientists have extensively investigated blood profiles and lungs of COVID-19 patients to understand the acute phase immunopathogenesis. These studies also helped researchers to identify diagnostic and protective markers. Two components of the blood, i.e., antibodies and T cells, play a critical role in the clearance of SARS-CoV-2 viruses and also protect from severe COVID-19.
Recent studies have reported long-term complications of the COVID-19 infection, which is known as Long COVID or post-acute sequelae of COVID-19 (PASC). These studies revealed that the immunopathogenesis of COVID-19 sustained beyond the recovery of pulmonary disease. In addition, uncharacteristic innate immune signaling cascades and inflammation were reported in COVID-19 convalescent patients. More studies are required to understand the pathophysiology associated with the initiation of aberrant innate immune activation at mucosal surfaces, which are in direct contact with the viral particles.
Transmission of SARS-CoV-2 primarily occurs through saliva, i.e., droplets generated by an infected individual while talking or coughing. This is why saliva is regarded as the optimal target to monitor alterations of the mucosal immune system by respiratory infection. In addition, several studies have reported the association between the oral/gastrointestinal mucosal alterations and the pathophysiology of PASC, highlighting the importance of saliva to identify the molecular mechanisms and early indicators of SARS-CoV-2.
A New Study
A new study posted to the bioRxiv* preprint server has focussed on the chronic inflammatory response during the early convalescent phase, i.e., two weeks post clinical symptoms of SARS-CoV-2. In this study, the blood and saliva samples were obtained from COVID-19 donors who visited the COVID clinic at the University of California, San Diego. Researchers investigated the association between biological and demographic factors. They evaluated the plasma antibody levels of the study cohort, which is considered to be the gold standard to analyze immune responses to viral infection. Scientists further examined the mucosal antibody response and proteomic alterations at systemic and mucosal levels.
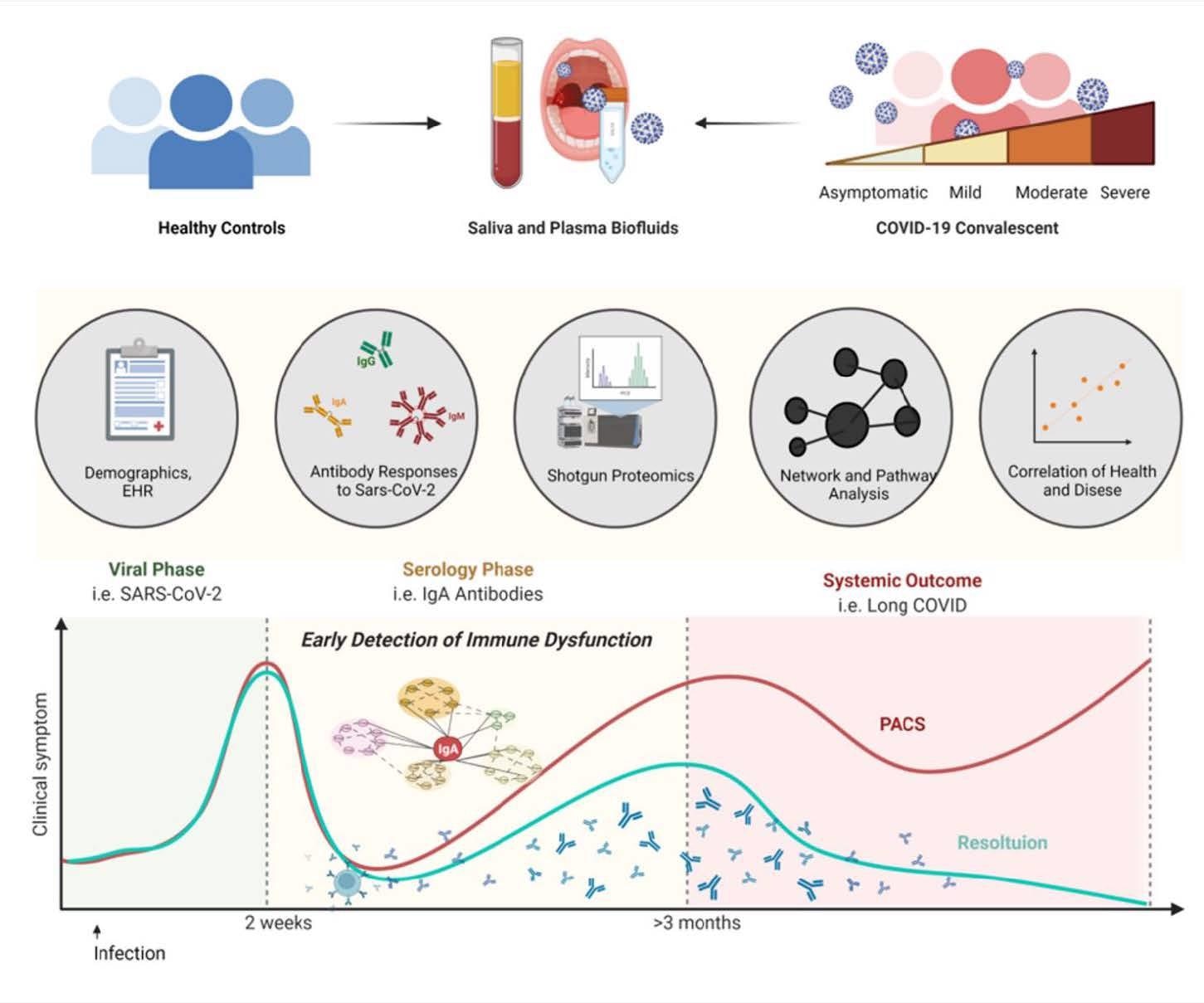 Study design
Study design

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Key Findings
The current study revealed the presence of strong inflammatory responses to the SARS-CoV-2 in convalescent plasma and saliva, even in the absence of clinical manifestation of PASC. Scientists detected aberrant immune responses and blood clotting dysfunctions in the study samples. They observed that the biofluids contained antibodies against the SARS-CoV-2 receptor-binding domain (RBD), spike protein (S1 and S2), and the nucleoprotein (NP). The authors reported the presence of RBD binding IgA in both saliva and plasma, RBD binding IgM in saliva, and S1 binding IgG in plasma. This result implies that saliva could be used to detect the presence of specific SARS-CoV-2 antibodies and immune responses.
Saliva also exhibited unique patterns associated with antibody and proteomic profiles that were different from plasma. Researchers also demonstrated that IgA response in COVID-19 convalescent individuals was significantly higher in saliva than plasma. However, IgG response followed an opposite trend. Importantly, the plasma samples provided information about the dysregulated blood clotting functions, while saliva samples conveyed greater information regarding immunopathogenesis, including information concerning dysregulated fibrin clot and dysregulated neutrophil pathways.
The authors assessed the biofluid samples via shot-gun proteomics to understand immunoglobulin patterns found during COVID-19, which affects the composition and function of the entire repertoire of human proteins. The authors stated that investigation of molecular patterns would help identify early signals associated with disease manifestations. Interestingly, the plasma proteomics of COVID-19 samples demonstrated dysregulated blood clot process, and salivary proteomics revealed responses linked to the innate immune compartments.
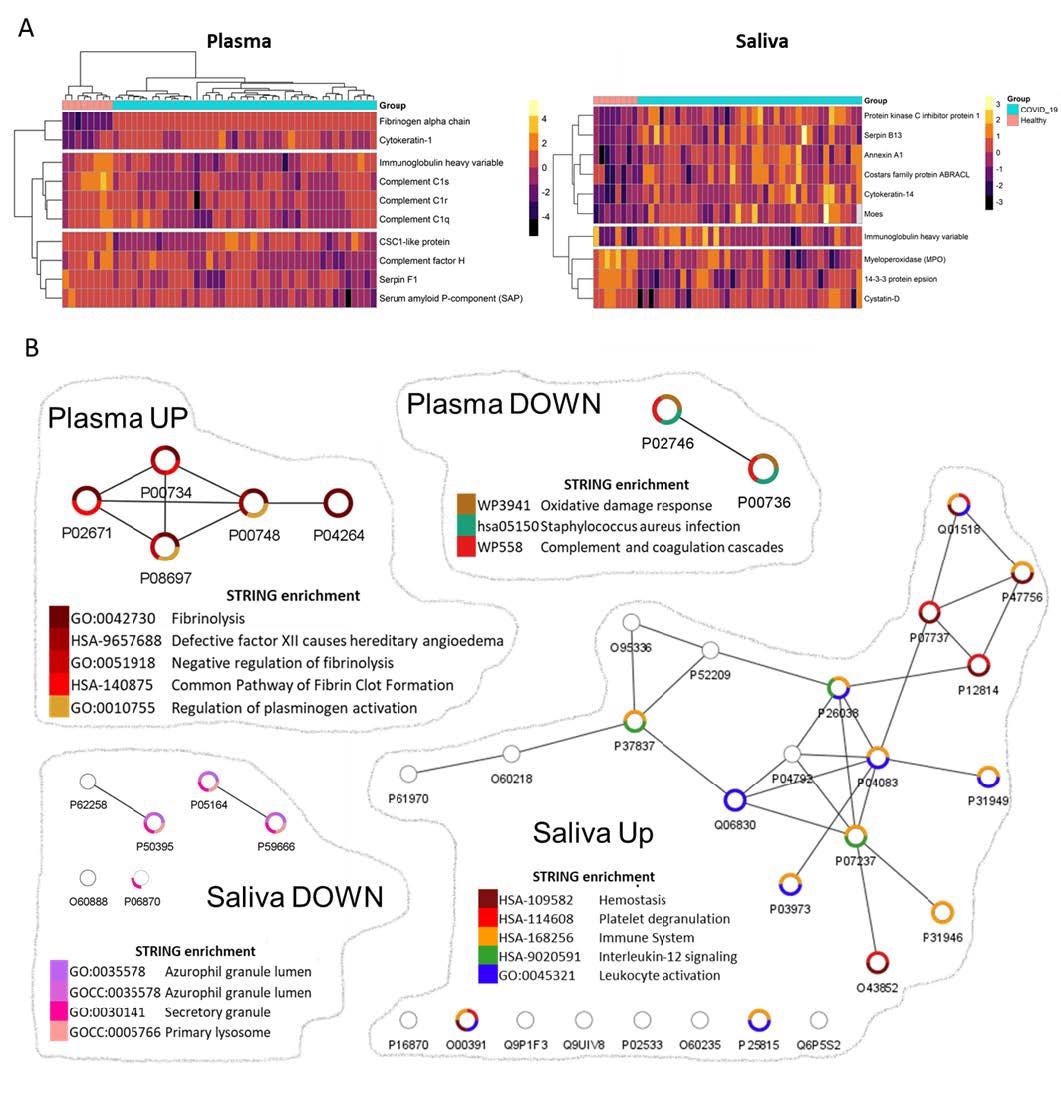
Network analyses depicted biological functions altered in convalescent plasma and saliva.
Saliva analysis of recovered COVID-19 individuals revealed that an increase in numerous proteins, such as antileukoproteinase and Matrix metalloproteinase-9, affects neutrophil functions or migration. Further, it also showed significant enhancement in transmembrane protease serine, responsible for activating the SARS-CoV-2 spike protein to enable the viral-cell fusion process. Proteomic analyses also exhibited significant correlations between salivary fibrinogen and salivary annexin-1. Scientists detected a strong IgA response in saliva to the SARS-CoV-2 infection, with a limited range of neutralizing activity. Interestingly, salivary IgAs secreted in polymeric form (dimeric and tetrameric) confer greater neutralizing activity compared to IgGs.
Limitations
One of the limitations of this study was its study cohort, which contained only SARS-CoV-2 infected individuals and no healthy donors. Additionally, the control samples came from the pre-COVID era, with matched demographics. Another limitation was that samples were collected at only a one-time point and the proteomic responses and antibody levels were not adjusted as per the baseline of each individual.
Conclusion
The current study reveals that saliva could serve as a potential immune biofluid. As saliva samples are easy to collect, they can be used for early detection and prevention of virus-induced chronic inflammatory sequelae. In the future, COVID-19 vaccines must be designed such that they can also activate a robust mucosal antiviral response, which could protect individuals against the disease.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.