The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant was largely responsible for a resurgence of coronavirus disease 2019 (COVID-19) cases in late 2021. In Israel, a campaign to implement a fourth vaccine or second booster was introduced by the Ministry of Health for those at high risk of infection from this variant who had already received three doses of vaccine. To this end, Israel approved a second booster dose on January 2, 2022, for people aged 60 and older, high-risk groups, and healthcare personnel who had received a first booster dose at least four months prior.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This decision to approve an additional booster dose was contentious due to a lack of epidemiological and large-scale clinical evidence supporting an additional COVID-19 vaccine dose. In fact, the effectiveness of providing the elderly with a second booster dose of the Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) vaccine in reducing rates of confirmed COVID-19 and severe disease was only recently published in preliminary short-term data. However, further research is needed to determine whether the additional booster dose is effective in preventing death from COVID-19.
About the study
In a recent study under consideration at a Nature Portfolio Journal and posted to the Research Square* preprint server, researchers determine whether there was any reduction in the death rate in elderly COVID-19 patients following the second booster dose.
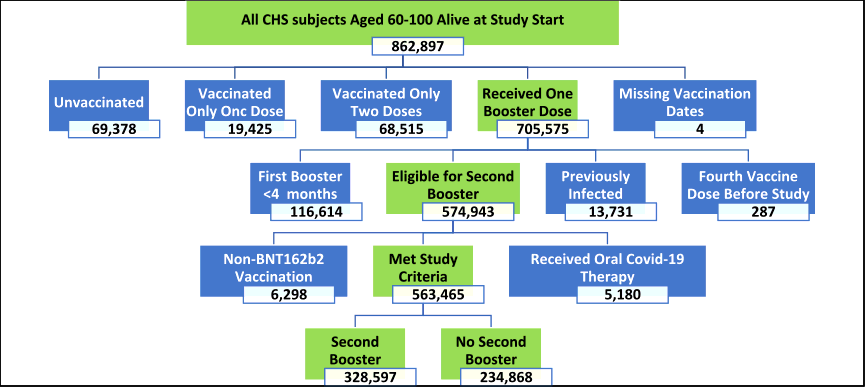
This observational and retrospective cohort study included data from Clalit Health Services (CHS) computerized medical records, which is a large healthcare organization that serves about 52% of Israel's population and about two-thirds of the country's elderly. All CHS members aged 60 to 100 who were eligible for a second-booster vaccine at the trial's start date were included in the study.
A total of 563,465 people met the inclusion criteria for the current study. The average age of the participants in the study was 73.0 years, with 53% of them being female. Hypertension, obesity, and diabetes were the most prevalent comorbidities.
Study participants were sorted into two groups including those who received a second booster and those who had only received the first booster dose. To provide time for antibodies to form effectively, participants were placed in the ‘second-booster' group seven days after receiving their second-booster vaccine dosage.
Assessment for Eligibility
Study outcomes
During the 40-day trial period, 328,597 individuals in the total population group received a second booster dose. The participation rate was much greater among those with a higher socioeconomic position, whereas ultra-Orthodox Jewish and Arab groups had lower uptake rates.
COVID-19-associated complications resulted in mortality in 92 of the second-booster participants and 232 of the first-booster recipients during the study period. In the second-booster group, the adjusted hazard ratio for death owing to COVID-19 was 0.22 as compared to the first-booster group.
The variables that passed Schoenfeld's Global Test for the proportional-hazards assumption were included in the Cox proportional-hazards regression model. Increased age, male sex, diabetes, chronic obstructive pulmonary disease, chronic heart failure, and ultra-Orthodox Jewish practice were all variables associated with death from COVID-19 that were reported in the Cox regression model.
For individuals between the ages of 60 to 69, COVID-19-associated death occurred in 5 of 111,776 individuals who had received the second booster and 32 of 123,786 who had received the first booster. In those between the ages of 70 to 79, COVID-19-associated death occurred in 22 of 134,656 individuals who had received the second booster and 51 of 74,717 individuals who had received the first booster.
For individuals between the ages of 80 to 100, COVID-19 associated death occurred in 65 of 82,165 individuals who had received the second booster and 149 of 36,365 individuals who had received the first booster.
Implications
Taken together, the current study found that following a second booster dose, death due to COVID-19 was considerably lower in people 60 years and older at least four months after receiving a BNT162b2 vaccination booster.
The findings from the current study demonstrated the possibility of preventing the most severe COVID-19 outcomes with a fourth booster vaccine dose in elderly patients. Thus, the current study provides evidence to assist decision-makers in appraising the value of administering the second booster to selected populations. Notably, studies with a longer follow-up period are still needed to determine the longevity of the efficacy and safety of a second booster COVID-19 vaccine dose.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Arbel, R., Sergienko, R., Friger, M., et al. (2022). Second Booster Vaccine and Covid-19 Mortality in Adults 60 to 100 Years Old. Research Square. doi:10.21203/rs.3.rs-1478439/v1. https://www.researchsquare.com/article/rs-1478439/v1.
- Peer reviewed and published scientific report.
Arbel, Ronen, Ruslan Sergienko, Michael Friger, Alon Peretz, Tanya Beckenstein, Shlomit Yaron, Doron Netzer, and Ariel Hammerman. 2022. “Effectiveness of a Second BNT162b2 Booster Vaccine against Hospitalization and Death from COVID-19 in Adults Aged over 60 Years.” Nature Medicine, April. https://doi.org/10.1038/s41591-022-01832-0. https://www.nature.com/articles/s41591-022-01832-0.