There have been increasing reports of changes to the menstrual cycle of younger individuals receiving coronavirus disease 2019 (COVID-19) vaccinations in the United Kingdom.
Notably, the majority of people experiencing these changes after immunization find that their period returns to normal by the next cycle. Furthermore, there is no indication that COVID-19 vaccination has a deleterious effect on female fertility.

Study: Effect of COVID-19 vaccination on menstrual periods in a prospectively recruited cohort. Image Credit: PandaStudio / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The United States National Institutes of Health (NIH) has committed $1.67 million to investigate the possible association between COVID-19 immunization and menstruation abnormalities in response to similar reports to the United States' vaccine safety surveillance network.
In one of these studies, researchers used data from a menstrual cycle tracking app that was prospectively logged. To this end, at least six consecutive cycles were reported by 3,959 Americans, 2,403 of whom were vaccinated and 1,556 served as a control group.
The first dosage of a COVID-19 vaccine had no influence on the time of the succeeding period in those who received only one dose per cycle; however, the second dose was related to a 0.45-day delay. Individuals who received two COVID-19 vaccine doses in a single cycle missed their period by 2.32 days. By two cycles following vaccination, cycle durations had reverted to normal in all groups.
COVID-19 effects on menstrual timing
In a recent study published on the preprint server medRxiv*, researchers report on the impact of COVID-19 vaccination on menstrual timing and flow in 79 women who have normal menstrual cycles or withdrawal bleeding after stopping hormonal contraception. These women were enlisted prior to receiving their first or second dose of the COVID-19 vaccine and were asked to keep a daily log of their vaginal bleeding, as well as any vaccine side effects.
The period following both the first and second doses of the vaccine arrived substantially later than expected. Especially in comparison to the day on which the individual expected their period, pre-vaccine periods happened on average 0.17 days early, while the period after dose one happened 2.3 days late and the period after dose two occurred 1.3 days late.
Interdose and post-vaccination cycles had periods that were 0.3 days late and 0.47 days early on average, respectively. Both of these values were not substantially different from the pre-vaccination average.
Although the findings from this study were similar to those of a major and well-designed study conducted in the United States, which similarly discovered delays in menstruation following a vaccination dosage, the delays discovered by the authors were slightly longer. As a result, they investigated whether individuals who had returned their journals were more likely to have noted any changes than those who had not, inflating the postvaccination alterations.
Thus, the authors conducted two sensitivity studies to address this issue. To this end, distributions that were consistent with no change were created and used to impute the data that would have been expected if the participants who said they stopped tracking their periods because they did not detect a difference had returned their diaries.
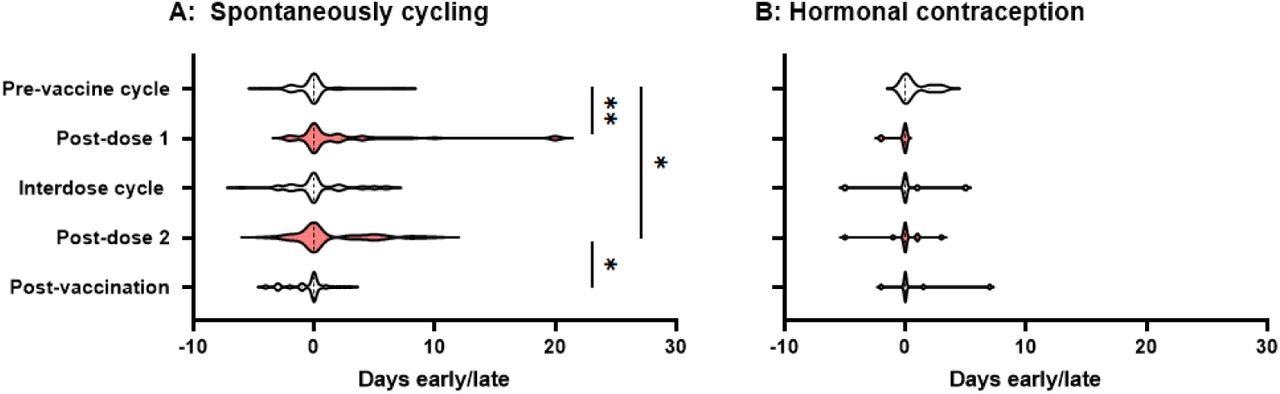
Violin plots showing the distribution by which periods or withdrawal bleeds began in pre-vaccine cycles, the cycle following dose 1 of the COVID-19 vaccine, interdose cycles, the cycle following dose 2 of the COVID-19 vaccine, and subsequent cycles, where “0” denotes the period or withdrawal bleed beginning on the expected day, negative numbers denote days early and positive numbers days late. Data for spontaneously cycling (A) and participants on hormonal contraception (B) are shown. * p’ < 0.05, * * P < 0.01.
At p' = 0.0013 and p' = 0.011, respectively, the differences between pre-vaccination cycle time and post-dose 1 and post-dose 2 timing remained significant. Even if none of the participants who did not return their journals perceived any difference, significant delays in the post-dose 1 and post-dose 2 periods persisted.
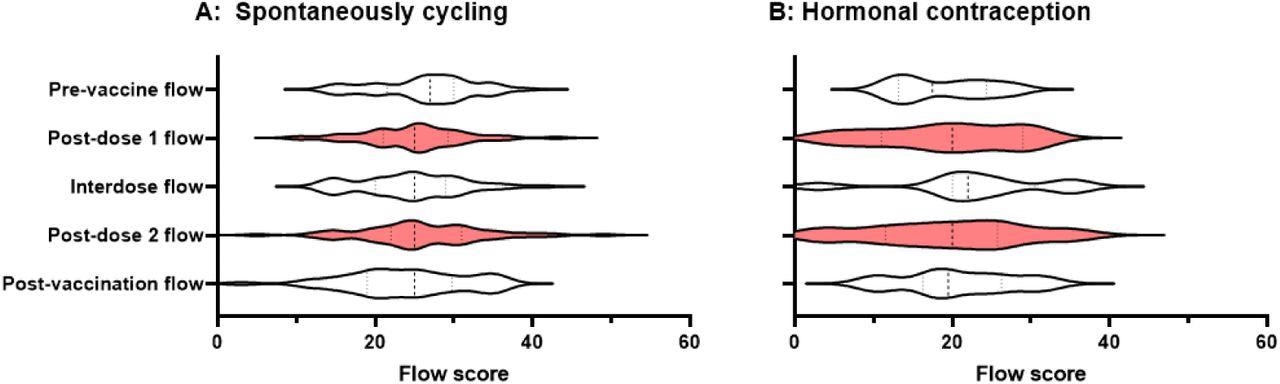
Violin plots showing the distribution of flow scores for periods or withdrawal bleeds in pre-vaccine cycles, the cycle following dose 1 of the COVID-19 vaccine, interdose cycles, the cycle following dose 2 of the COVID-19 vaccine, and subsequent cycles. Data for spontaneously cycling (A) and participants on hormonal contraception (B) are shown.
Immune activity may temporarily disrupt the hypothalamic-pituitary-adrenal axis or immune cells in the uterine lining, which regulate the breakdown and renewal of this tissue. In either instance, these changes may be regarded as menstruation alterations that are analogous to other vaccine-induced short-term and transitory adverse effects.
As a result, menstrual alterations could be more common in people who have had common side effects. To test this idea, the authors looked for relationships between self-reported side effect scores and future period timing or flow score. No significant connections were detected. As a result, the authors were unable to discover evidence that menstrual abnormalities are linked to other prevalent vaccine side effects in this small group.
Implications
Both the first and second doses of COVID-19 vaccines were related to a modest delay in the succeeding period in this small and prospectively selected sample. Interdose and post-vaccination cycles did not show this delay, thereby implying that any changes are transitory.
Taken together, changes in menstrual flow were not detected in this study. Although these results are encouraging, more research in larger prospectively recruited groups is needed to identify how immunization impacts menstruation parameters more precisely.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources