Scientists from the United States have recently investigated the impact of pre-existing health conditions on the robustness and durability of immune responses induced by mRNA-based coronavirus disease 2019 (COVID-19) vaccination.
The study was conducted on a group of veterans and healthcare workers. The findings reveal that the immunity induced by primary vaccination is affected by older age and specific comorbidities. However, the booster vaccination can induce universally robust and unaffected immunity. The study is currently available on the medRxiv* preprint server while awaiting peer review.
 Study: Clinical Variables Correlate with Serum Neutralizing Antibody Titers after COVID-19 mRNA Vaccination in an Adult, US-based Population. Image Credit: Suzanne Tucker / Shutterstock
Study: Clinical Variables Correlate with Serum Neutralizing Antibody Titers after COVID-19 mRNA Vaccination in an Adult, US-based Population. Image Credit: Suzanne Tucker / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The US Food and Drug Administration (FDA) has approved three COVID-19 vaccines for emergency use in the US, including two mRNA-based vaccines with high safety and efficacy profiles. These vaccines contain full-length spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as an immunogen.
In clinical trials and real-world situations, these vaccines have shown high efficacy in inducing robust neutralizing antibody titers, justifying their ability to protect against SARS-CoV-2 infection and symptomatic disease.
However, a declining vaccine efficacy has been observed worldwide within 6 months after the completion of primary vaccination, which includes two doses of the vaccine given at a fixed interval. Therefore, to improve vaccine efficacy, the public health authorities of many countries have decided to immunize at-risk populations with a third booster dose.
In the current study, the scientists have investigated whether pre-existing health conditions impact the robustness and durability of anti-SARS-CoV-2 immunity induced by primary and booster COVID-19 vaccination.
Study design
The study was conducted on 91 veterans and 33 healthcare workers who had received two doses of the mRNA-based COVID-19 vaccine developed by Pfizer/BioNTech. In addition, the study included 36 participants who had received the booster vaccination six months after the completion of the primary vaccination.
To measure anti-spike neutralizing antibody titers, blood samples were obtained from the participants before first and second vaccination and one month, three months, and six months after the second vaccination. The neutralizing titer was measured one month after the third booster dose in boosted participants.
Robustness and durability of vaccine-induced antibody response
The highest titer of anti-spike neutralizing antibodies was observed one month after the second vaccination, 14-fold higher than the pre-vaccination titer. Afterward, the titers declined gradually over the period of six months post-vaccination. At month 6, the average titer was only 3-fold higher than the pre-vaccination titer. The participants with a robust initial immune response to vaccination exhibited higher efficiency in maintaining the response for longer.
After booster vaccination, a marked increase in antibody titer was observed. Specifically, the average titer at month 1 post-booster vaccination was 52-fold higher than the pre-vaccination titer.
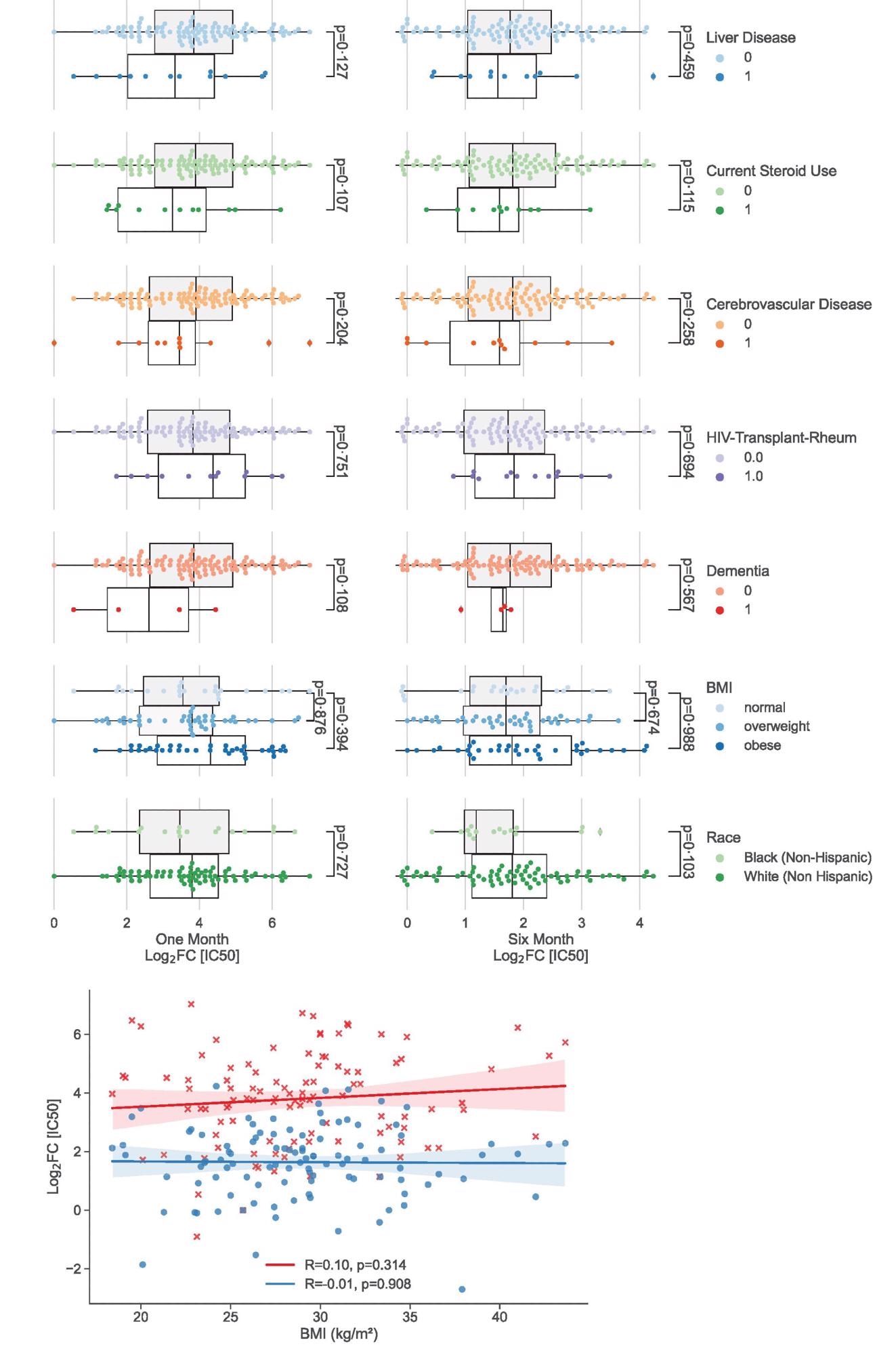
Univariate analysis showing clinical factors not associated significantly with neutralizing antibody peak and duration. (a) Categorical analysis between clinical variables not associated (p>0.10) with vaccination response at one month (left) and six months (right) following 2nd dose of the vaccine. (b) Scatterplot illustrating continuous variables: BMI plotted against vaccination response at one month (red crosses) and six months (blue circles). Colored lines represent lines of best fit with shading showing 95% confidence intervals.
Impact of comorbidities on vaccine-induced antibody response
A significant correlation was observed among demographic characteristics between older age and reduced robustness of primary vaccination-induced antibody response. However, no impact of age on the durability of response was observed. Regarding gender, female participants showed significantly more robust antibody responses compared to male participants.
Among various comorbidities studied, diabetes, malignancy, and chronic heart disease showed significant independent correlations with reduced robustness of antibody response. However, except for diabetes, no impact of other comorbidities was observed on the duration of response. In addition, participants with poor kidney functions showed a lower antibody response to primary vaccination.
Further statistical analysis considering multiple factors indicated that poor kidney functions, diabetes, and current use of steroids correlate significantly with reduced duration of antibody response to primary vaccination. In addition, older age and malignancy were found to impact the duration of response. A non-significant impact of liver diseases was also observed on the robustness of response.
Importantly, no impact of studied demographics and comorbidities was observed on the robustness and duration of antibody response to booster vaccination. This indicates that the booster dose can induce a universally robust antibody response that remains unaffected by demographic and clinical factors.
Study significance
The study identifies several demographic and clinical factors that negatively impact the robustness and durability of antibody response to primary COVID-19 vaccination. These factors include older age, diabetes, heart and kidney disease, and malignancy. Importantly, no impact of these factors has been observed on the booster vaccination-induced antibody response.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zhao M. 2022. Clinical Variables Correlate with Serum Neutralizing Antibody Titers after COVID-19 mRNA Vaccination in an Adult, US-based Population. MedRxiv. https://www.medrxiv.org/content/10.1101/2022.04.03.22273355v1
- Peer reviewed and published scientific report.
Zhao, Min, Rebecca Slotkin, Amar H Sheth, Lauren Pischel, Tassos C Kyriakides, Brinda Emu, Cynthia McNamara, et al. 2022. “Serum Neutralizing Antibody Titers 12 Months after Coronavirus Disease 2019 Messenger RNA Vaccination: Correlation to Clinical Variables in an Adult, US Population.” Clinical Infectious Diseases 76 (3): e391–99. https://doi.org/10.1093/cid/ciac416. https://academic.oup.com/cid/article/76/3/e391/6593314.