The team found that two doses of the intradermally administered HexaPro S protein produce a potent immune response and strong neutralization of the wild-type as well as the Alpha and Delta variants of SARS-CoV-2.
In addition, the study validates the competence of a relatively novel vaccine delivery approach, which is an injection-free High-density microarray patch (HD-MAP), the use of which can ensure vaccine distribution to low-income and middle-income countries.
How will HexaPro S protein delivery make a difference?
The surface spike “S” protein is the major antigenic target for the coronavirus disease 2019 (COVID-19) vaccines owing to its property of binding to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells to facilitate viral entry into the cell. Antibodies generated against the S protein can prevent viral attachment to the host cell and neutralize the virus.
Dr. David A. Muller and Dr. Christopher L. D. McMillan co-led the team to assess the immunogenic potential of a commercially available HexaPro S protein (ExcellGene, Switzerland), which is modified to encompass six stabilizing substitutions against SARS-CoV-2. The modified commercial S protein, owing to its proline substitutions, exhibits certain advantages over its parental construct in terms of higher expression levels, being more thermostable, and being able to resist at least three freeze-thaw cycles.
The downside of currently available SARS-CoV-2 vaccines, which hinder their distribution to the poorer countries, is the requirement of ultra-low temperatures for storage. An urgent need, therefore, exists for vaccines that are thermostable and simultaneously are easy to administer, permitting global ease of access.
One such approach to improving vaccine stability and ease of distribution is the high-density microarray patch, HD-MAP. The HD-MAP is a micro-projection array containing 250 μm long projections (n=5000) which are coated with the specific vaccine antigen, and a patch is then applied to the skin with a spring-loaded applicator, delivering the vaccine directly to the epidermal and dermal layers of the skin. These layers are immune-rich i.e. abundant in antigen-presenting cells (APC), leading to a superior immunogenic response.
Moreover, viral vectors used in vaccines have been shown to remain immunogenic for up to 10 weeks at higher temperatures of 37°C on such microarray patches.
This needle-free approach can therefore offer advantages in terms of improved thermostability, enhanced antigenicity and simplified storage/transport, circumventing the challenges caused by cold-chain failures. Moreover, the patches are easy to apply, thus negating the need for trained healthcare workers.
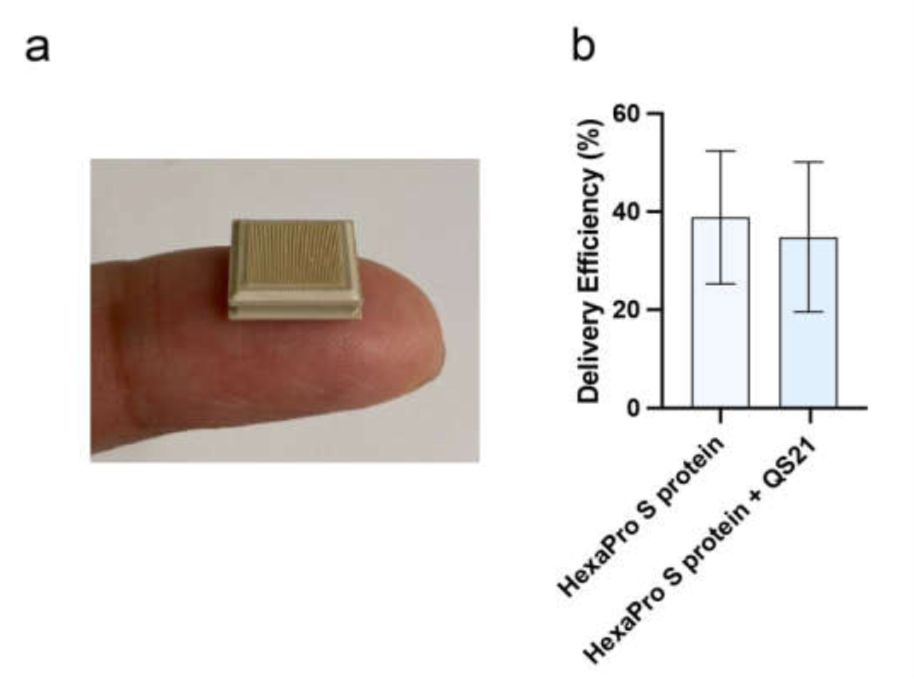 Figure 1: HexaPro S protein-based vaccine and its application using HD-MAP. (a) HD-MAPs containing 5000 solid polymer microprojection arrays to deliver vaccine into the cutaneous layer of the skin. (b) Delivery efficiency of antigen into the skin using HD-MAPs coated with SARS CoV-2 HexaPro S protein and QS-21-adjuvanted SARS CoV-2 HexaPro S protein (n = 5, each) was measured by comparing the remaining protein from the delivered HD-MAPs to undelivered HD-MAPs using capture ELISA.
Figure 1: HexaPro S protein-based vaccine and its application using HD-MAP. (a) HD-MAPs containing 5000 solid polymer microprojection arrays to deliver vaccine into the cutaneous layer of the skin. (b) Delivery efficiency of antigen into the skin using HD-MAPs coated with SARS CoV-2 HexaPro S protein and QS-21-adjuvanted SARS CoV-2 HexaPro S protein (n = 5, each) was measured by comparing the remaining protein from the delivered HD-MAPs to undelivered HD-MAPs using capture ELISA.
What did the researchers do?
Initially, after the commercial procurement of the SARS-CoV-2 HexaPro S protein, the team evaluated the purity, structural integration, and antigenic potential of the protein. The HexaPro S protein (with and without adjuvant QS-21) was then coated on to the HD-MAP to perform immunization studies in mice.
The team also compared the efficiency of HexaPro S protein with or without the adjuvant (QS-21) via two delivery methods, HD-MAP application, and intradermal (ID) injection. Two doses were administered in BALB/c mice 21 days apart, and serum was collected on day 20 and day 42 post-first vaccination to measure IgG antibody and virus-neutralization titers.
What did the researchers find?
S-specific IgG levels post-second dose in the HD-MAP-delivered HexaPro S protein group were significantly higher than in the corresponding ID delivered group. Also, IgG antibodies were not at all observed in the ID group after the first dose.
Irrespective of the delivery method, the IgG antibody titers in the adjuvanted HexaPro S protein groups were significantly higher than in the unadjuvanted HexaPro S protein groups. This was true for all the three variants tested i.e. wild-type, Alpha, and Delta SARS-CoV-2.
Virus-neutralizing antibody titers were also observed to be raised to similar levels across the three variants, suggesting that the broad immune response elicited by HexaPro S protein combined with QS-21 is able to overcome the mutations in the Alpha and Delta variants”, highlights the team.
For the unadjuvanted groups, mice that received the HD-MAP delivered HexaPro S protein had significantly higher virus-neutralizing antibodies as compared to the ID delivered group.
Notably, after the first dose, even the unadjuvanted HexaPro S protein, delivered via the HD-MAP, resulted in significantly higher IgG and neutralizing potential as compared to the ID delivered adjuvanted HexaPro S protein group.
The findings provide evidential proof of the superiority of the HD-MAP intradermal delivery approach in comparison to the traditional needled ID injection method. This study also highlights the influence of adjuvants when delivering the protein subunit vaccines.
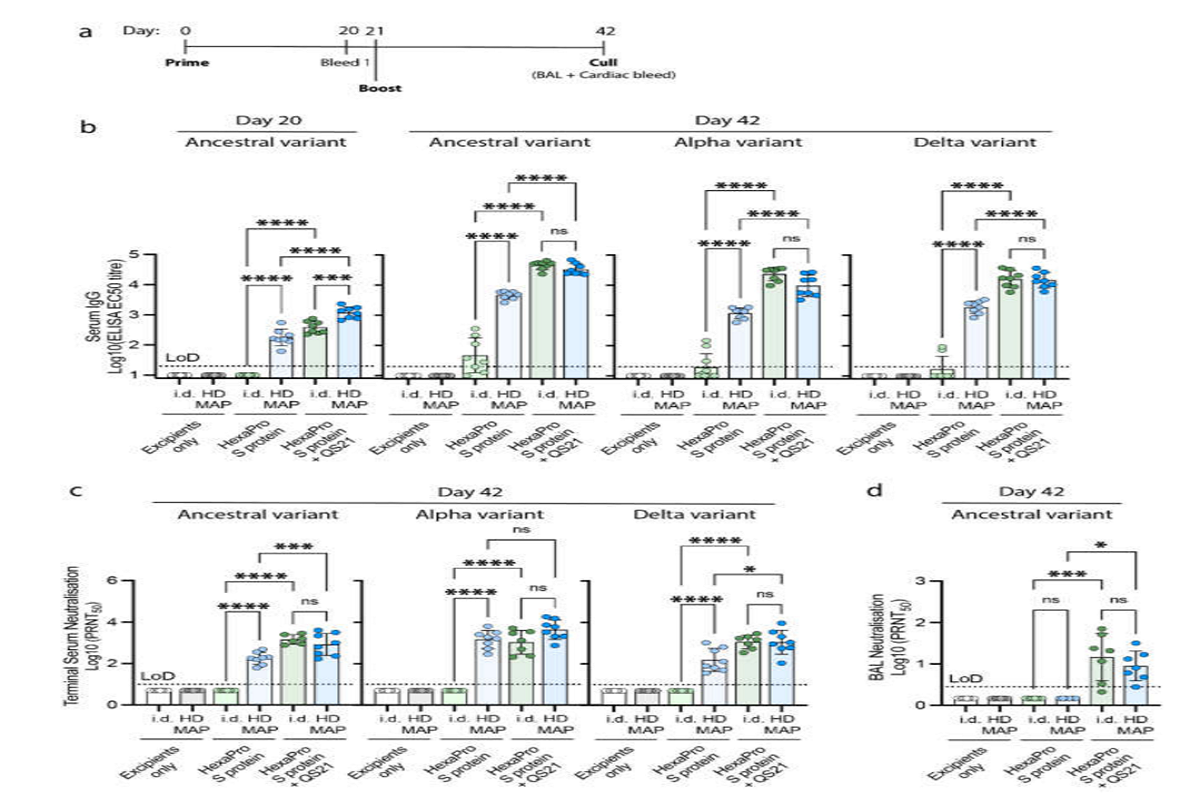 Figure 2: Immune responses in BALB/c mice following the vaccination with excipients (negative control), HexaPro S protein, and HexaPro S protein + QS-21 via intradermal injection (i.d.) or HD-MAP application (n = 8, each), (a) vaccination schedule. Serum was collected after primary immunization and first boost (on day 20 and 42, respectively) and analyzed for (b) serum IgG antibody titers against HexaPro S protein and HexaPro S protein derived from Alpha and Delta variants by ELISA. Serum and bronchoalveolar lavage (BAL) collected on day 42 were analyzed for (c) serum virus neutralization by plaque reduction neutralization test (PRNT) against the parental SARS-CoV-2 isolate, an Alpha variant, and a Delta variant, and (d) BAL virus neutralization by PRNT against ancestral SARS-CoV-2 variant, respectively. Each point represents an individual biological replicate (mouse) performed on a single ELISA assay; bars represent the average antigen-specific IgG antibody titers (EC50); error bars represent the SD; the LoD line represents the assay limit of detection. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison post hoc test ((*) p < 0.05, (***) p < 0.001, (****) p < 0.0001 and non-significant (ns)).
Figure 2: Immune responses in BALB/c mice following the vaccination with excipients (negative control), HexaPro S protein, and HexaPro S protein + QS-21 via intradermal injection (i.d.) or HD-MAP application (n = 8, each), (a) vaccination schedule. Serum was collected after primary immunization and first boost (on day 20 and 42, respectively) and analyzed for (b) serum IgG antibody titers against HexaPro S protein and HexaPro S protein derived from Alpha and Delta variants by ELISA. Serum and bronchoalveolar lavage (BAL) collected on day 42 were analyzed for (c) serum virus neutralization by plaque reduction neutralization test (PRNT) against the parental SARS-CoV-2 isolate, an Alpha variant, and a Delta variant, and (d) BAL virus neutralization by PRNT against ancestral SARS-CoV-2 variant, respectively. Each point represents an individual biological replicate (mouse) performed on a single ELISA assay; bars represent the average antigen-specific IgG antibody titers (EC50); error bars represent the SD; the LoD line represents the assay limit of detection. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison post hoc test ((*) p < 0.05, (***) p < 0.001, (****) p < 0.0001 and non-significant (ns)).