Globally, there is a need for an equitable coronavirus disease 2019 (COVID-19) vaccine supply. The NVX-CoV2373 vaccine showed promise in phase IIb and III clinical trials in the United Kingdom (UK) and South Africa, likewise in a phase III trial in the United States (US) and Mexico. Accordingly, it earned emergency use authorization (EUA) in multiple countries around the globe.
However, a detailed understanding of the immune responses elicited in response to the NVX-CoV2373 vaccine is currently lacking.
About the study
In the present study, researchers measured CD4+ and CD8+ T cell responses to NVX-CoV2373 and the effect of cross-reactive CD4+ T cells. They performed an activation-induced marker (AIM) assay to measure SARS-CoV-2 S-specific CD4+ T cells.
Notably, cross-reactive CD4+ T cells are memory T cells generated in response to infections with commonly circulating human coronaviruses (HCoVs), including OC43, 229E, and NL63, and are capable of cross-reacting with shared SARS-CoV-2 epitopes.
The researchers isolated peripheral blood mononuclear cells (PBMCs) from blood samples of all the study participants on days 0, 7, and 28. Of the total 32 participants, 27 had received five μg of NVX-CoV2373 on days 0 and 21, five volunteers received five μg of NVX-CoV2373 on day 0, and a placebo on day 21, and four individuals received only a placebo.
The team assessed the functionalities of NVX-CoV2373-induced CD4+ T cell responses by identifying S-specific circulating follicular helper T cells (cTFH) and via intracellular cytokine staining (ICS) of S-specific CD4+ T cells. Likewise, the researchers assessed the relationship between T cells and antibody responses following the NVX-CoV2373 vaccination.
The authors performed an enzyme-linked immunosorbent assay (ELISA) to measure the immunoglobulin G (IgG) titers. Additionally, they performed microneutralization assays and human angiotensin-converting enzyme 2 (hACE2) binding inhibition assays to evaluate SARS-CoV-2 neutralizing antibodies (nAbs).
Study findings
On day 0, five of 32 participants had detectable SARS-CoV-2 S-specific CD4+ T cells, indicative of pre-existing T cell memory. By day 7, after the first and the second vaccination, 50% and 81% of donors developed S-specific CD4+ T cell responses of comparable magnitude, respectively.
TFH cells are crucial for antibody responses following vaccination. The authors observed that 36% of vaccinees, one week after the first vaccination, and 44% of vaccinees, seven days after the second vaccination, had significantly increased cTFH cells relative to day 0.
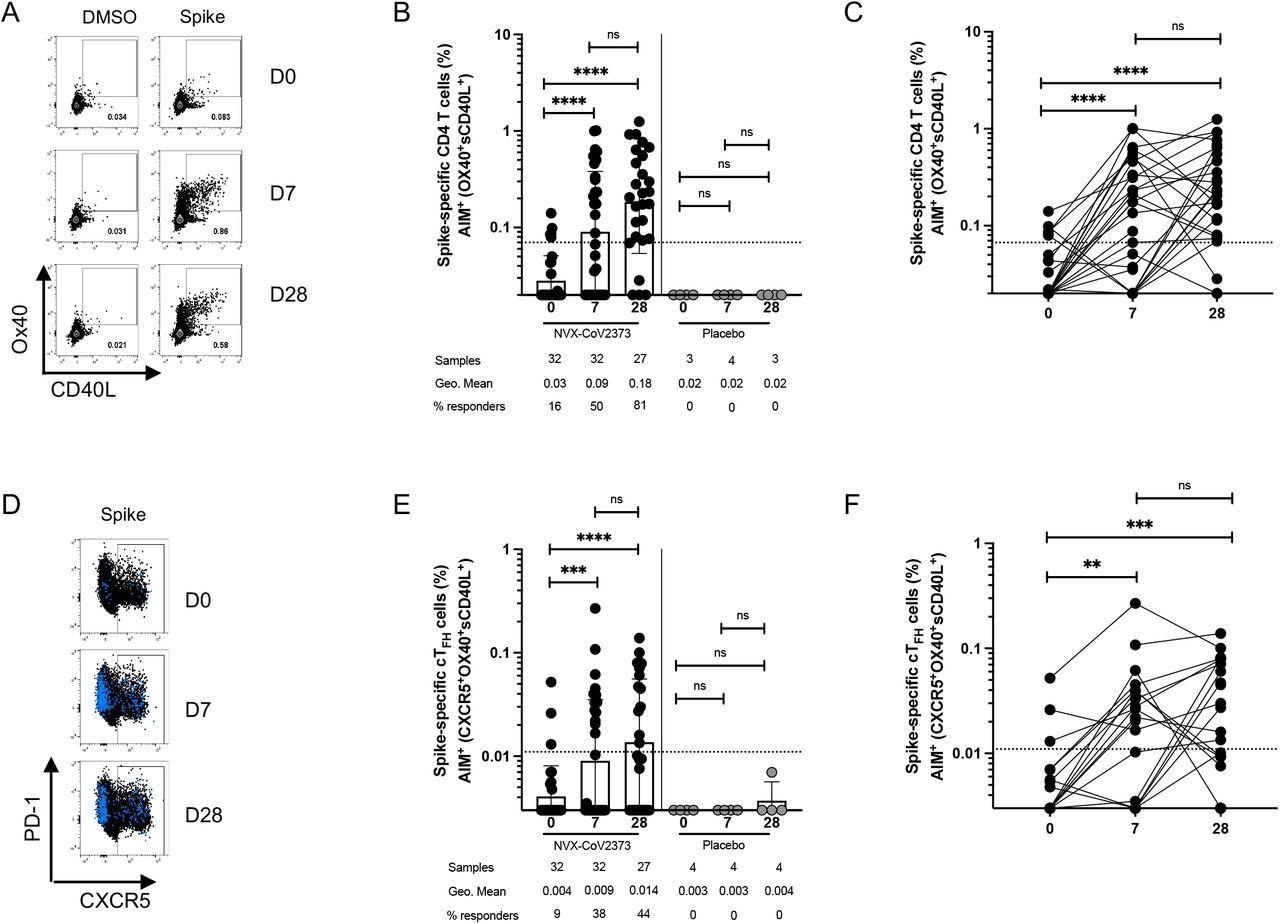
Spike-specific CD4+ T cells following NVX-CoV2373 vaccination. (A) Representative FACS plots of AIM+ (sCD40L+OX40+) CD4+ T cells at D0, D7 and D28 post-vaccination. (B-C) Spike-specific AIM+ CD4+ T cell responses in vaccinees (black dots) and placebo controls (grey dots). The same data is graphed as grouped (B) and paired comparisons (C). (D) Representative FACS plots of AIM+ CD4+ CXCR5+ cTFH cells (blue dots are AIM+ CD4+ T cells overlaid on total CD4+ T cells in black). (E-F) Spike-specific AIM+ cTFH cell responses in vaccinees and placebo controls. The same data is graphed as grouped (E) and paired comparisons (F). Dotted line indicates the limit of quantitation (LOQ) for the assay, and is calculated as the geometric mean of all sample DMSO wells multiplied by the geometric SD factor. % responders are calculated as responses ≥ LOQ divided by the total samples in the group.
Interferon-gamma (IFNγ), tumor necrosis factor-alpha (TNFα), and interleukin 2 (IL-2)-secreting CD4+ T cell frequencies had significantly increased within a week after the first NVX-CoV-2373 dose and increased further after the second dose.
NVX-CoV2373 vaccination also induced polyfunctional CD4+ T cell cytokine responses. The study data indicated that S-specific CD4+ T cells elicited in response to the NVX-CoV-2373 vaccine comprised classical type 1 T helper cells (Th1) and cTFH cells that are necessary for supporting antiviral and antibody responses.
AIM detected a modest S-specific CD8+ T cell response seven days after the first vaccination in 9% of the study participants. However, within a week after the second NVX-CoV-2373 dose, 20% of the study participants had a similar magnitude of CD8+ T cell responses.
While CD4+ T cell populations significantly correlated with nAbs after both the first and second vaccination, S-specific cTFH cell frequencies did not correlate with the anti-S IgG titers or nAbs.
Conclusions
In contrast to live-attenuated or inactivated vaccines, protein-based vaccines have historically proven to be poor inducers of CD8+ T cell responses in humans. However, the NVX-CoV-2373 vaccine does induce modest S-specific CD8+ T cell responses in a subset of individuals, as demonstrated by AIM.
ICS assays showed similar results, with IFNγ CD8+ T cells detected after both first and second vaccinations. The observed CD8+ T cell responses were less polyfunctional but multifunctional. Future studies are warranted to validate whether the observed CD8+ T cell responses depend on the CD4+ T cell responses.
The authors also observed that pre-existing cross-reactive memory CD4+ T cells did not affect NVX-CoV2373-induced SARS-CoV-2 S-specific T cell responses. Hence, future studies should directly compare the pre-existing T cells across a range of COVID-19 vaccine platforms to elucidate the role of these cells in vaccine responses.
The NVX-CoV2373 vaccination also recalled S-specific memory CD4+ T cells and B cells in baseline seropositivity developed on previous exposure to SARS-CoV-2. Accordingly, these individuals showed an enhanced nAb response after the first dose of NVX-CoV2373.
Finally, compared to messenger ribonucleic acid (mRNA) vaccines, NVX-CoV2373 potentially induced relatively broad and long-term humoral and cellular immune responses against SARS-CoV-2 in humans.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
NVX-CoV2373 vaccination induces functional SARS-CoV-2–specific CD4+ and CD8+ T cell responses, Carolyn Rydyznski Moderbacher, Christina Kim, Jose Mateus, Joyce Plested, Mingzhu Zhu, Shane Cloney-Clark, Daniela Weiskopf, Alessandro Sette, Louis Fries, Gregory Glenn, Shane Crotty, bioRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.04.08.487674, https://www.biorxiv.org/content/10.1101/2022.04.08.487674v1
- Peer reviewed and published scientific report.
Rydyznski Moderbacher, Carolyn, Christina J. Kim, Jose Mateus, Joyce S. Plested, Mingzhu Zhu, Shane Cloney-Clark, Daniela Weiskopf, et al. 2022. “NVX-CoV2373 Vaccination Induces Functional SARS-CoV-2—Specific CD4+ and CD8+ T Cell Responses.” Journal of Clinical Investigation, August. https://doi.org/10.1172/jci160898. https://www.jci.org/articles/view/160898.