
 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
Genetic variants of SARS-CoV-2 persist to evolve as the virus spreads over the globe, and each mutated viral variant has the potential to elude adaptive immunity and renew the coronavirus disease 2019 (COVID-19) pandemic. SARS-CoV-2 is an enveloped ribonucleic acid (RNA) virus with a positive-sense, single-stranded genome and spike (S) glycoproteins on its surface.
The SARS-CoV-2 S protein is a highly immunogenic antigen that induces a significant neutralizing antibody response and is encoded by the substantially efficient COVID-19 messenger RNA (mRNA) vaccines. Since the S protein plays a vital role in SARS-CoV-2 host entry and immunity, its mutations must be closely monitored.
COVID-19 patients infected with the SARS-CoV-2 B.1.617.2 lineage (Delta variant) experience a rapid initial infection with a superior viral load to the previous viral variants. Further, recent reports suggested that the SARS-CoV-2 Delta strain S protein PPs elicit a quicker initial infection rate to target cells than those exhibiting S of other SARS-CoV-2 variants.
About the study
In the present study, the scientists searched for an ultrastructural link to the elevated initial SARS-CoV-2 infection rate of the Delta variant PPs. For this, the team analyzed the structural properties of murine leukemia viruses (MLVs)-derived PPs harboring S proteins of various SARS-CoV-2 variants through negative stain transmission electron microscopy (TEM). The evaluated CoV S proteins were from the SARS-CoV-2 D614G mutant strain, Delta variant, Alpha variant, Delta AY.4.2 sublineage, and Omicron BA.1 sublineage. Additionally, the team also evaluated bald PPs lacking SARS-CoV-2 S proteins.
Human embryonic kidney 293T (HEK293T) cells were used for cell cultures and the generation of SARS-CoV-2 variants S protein PPs. The concentration and size of SARS-CoV-2s S PPs were profiled at four hours, 24 hours, and one week using nanoparticle tracking analysis (NTA). Additional assessments performed in this study include cryogenic electron microscopy (cryo-EM) and flow cytometry.
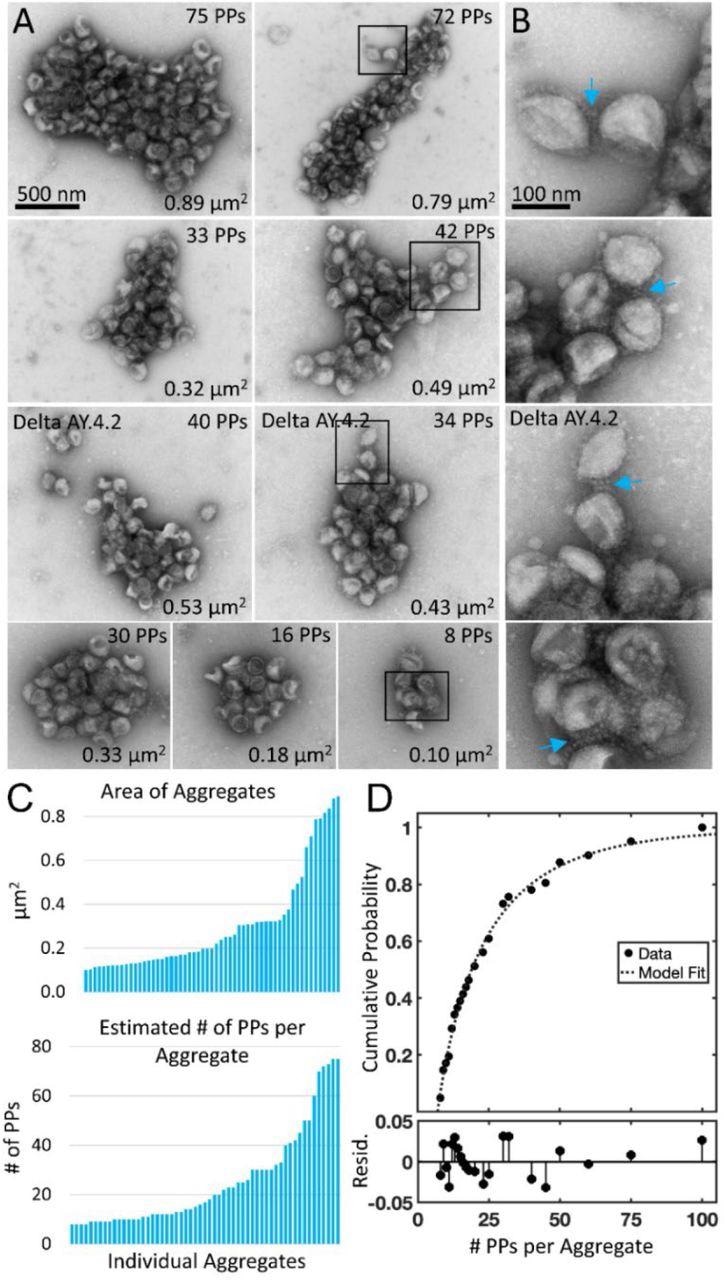
Negative stain TEM of Delta and Delta AY.4.2 aggregates observed 4 hours after harvest from producer cells. (A) Delta and Delta AY.4.2 PP aggregates representing range of sizes at the 4-hour timepoint. The number of PPs estimated per aggregate is indicated in the upper right corner of each image and the area occupied by the aggregate indicated in the lower right corner. Two Delta AY.4.2 PP aggregates are labeled, all others shown are Delta PPs (unlabeled). (B) Areas boxed in A are shown enlarged to the right of each image. Blue arrows highlight spike tip interactions occurring between PPs at the periphery of aggregates. (C) Graphs show ordered lists of the estimated number of PPs and areas of aggregates. (D) Truncated Weibull Distribution for Delta Variant. Negative stain data at 4 hours was fit by the general model: f(x) = (wblcdf(x,a,b)-wblcdf(7,a,b))/(1-wblcdf(7,a,b)) where wblcdf is the Weibull Distribution and x is the PPs per aggregate, 7 is the truncation value, and a and b are the Weibull scale and shape parameters. Coefficients (with 95% confidence bounds) are a = 11.47 (8.35, 14.60) and b = 0.68 (0.57, 0.79). Goodness of fit measure Sum of Squared Error (SSE) = 0.009 and Standard Error of Regression (RMSE) = 0.021, and Resid. are the residuals or differences between the model fit and the data.
Results
The study results illustrated that the SARS-CoV-2 Delta and Delta AY.4.2 S PPs established distinct aggregates, as evidenced by NTA, flow cytometry, negative stain TEM, and cryo-EM assessment. On the contrary, none of these parameters indicated aggregation in bald PPs and viral particles pseudotyped with S from Omicron, Alpha, and D614G mutant strain.
The observed aggregation of the Delta PPs was most probably due to a unique characteristic of the Delta AY.4.2 and Delta S, as all SARS-CoV-2 PPs were handled under similar circumstances, prepared concurrently, and elements that could lead to aggregation were avoided. In addition, the fact that Delta AY.4.2 and Delta PPs persisted to aggregate in solution after being kept at 4°C showed that aggregation occurs following budding from the producer cell. Nevertheless, the interaction between PPs might also arise during budding or biosynthesis from the producer cells.
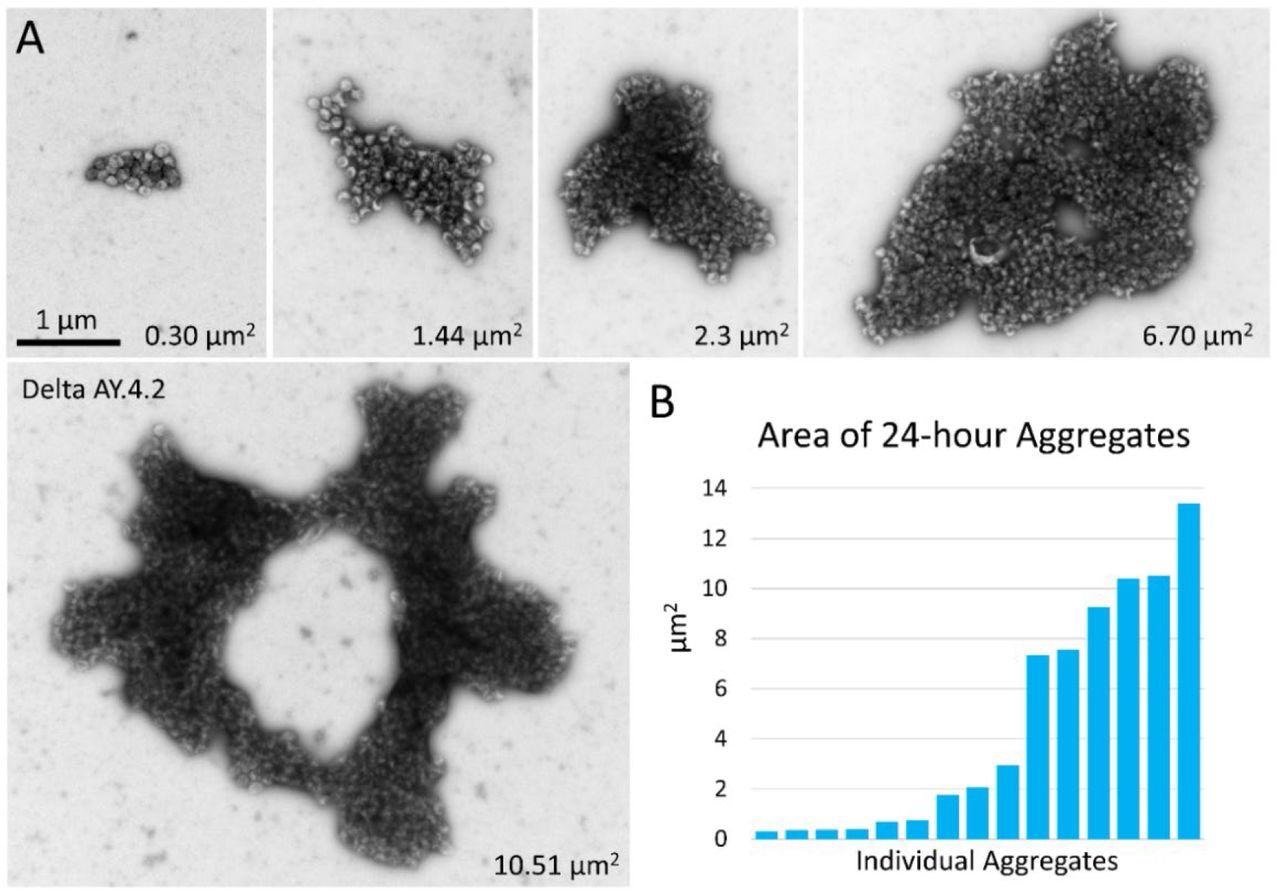
Negative stain TEM of Delta and Delta AY.4.2 aggregates 24 hours after harvest from producer cells. (A) Images of aggregates of Delta and Delta AY.4.2 PPs representing range of sizes observed after storage overnight at 4°C. The area occupied by each aggregate is indicated in the lower right corner. One donut-shaped Delta AY.4.2 aggregate is labeled, all other aggregates are Delta PPs (unlabeled). All images are scaled the same and scale bar is indicated. (B) Graph shows an ordered list of the areas of Delta aggregates.
Additionally, the potential of Delta to aggregate might be influenced by the number of virus particles present in each setting. The continued aggregation of Delta PP was congruent with a mass action mechanism for Delta PP clustering.
SARS-CoV-2 variant PPs were produced using a 19 amino acid (aa) C-terminal truncated version of each variant S, which could enhance the PP infectivity and quantity of S integrated into the PP envelope. Truncations in the cytoplasmic tail might alter the function and structure of the S ectodomain. Although each SARS-CoV-2 variant S had a similar truncation, it was plausible that the truncation affected the Delta S ectodomain differently, granting the aggregation feature.
Further, three unique and identical mutations at the E156G, R158, and F157 residues were found in the N-terminal domain (NTD) of Delta AY.4.2 and Delta which might be adequate to confer the aggregation characteristics to Delta. Furthermore, the distinct combination of mutations on the Delta S compared to the other SARS-CoV-2 variants analyzed could also induce the aggregation synergistically.
Notably, the clustering of Delta PPs was responsible for the higher and faster initial SARS-CoV-2 infection with Delta PPs in the entry assessments. Viral aggregations enhance viral fitness by safeguarding virions from environmental hazards and favors the simultaneous delivery of multiple viral genomes, also called collective infection. A collective infection could promote initial SARS-CoV-2 infection in some settings. Further, too large or too few virions would not impart enhanced infectivity.
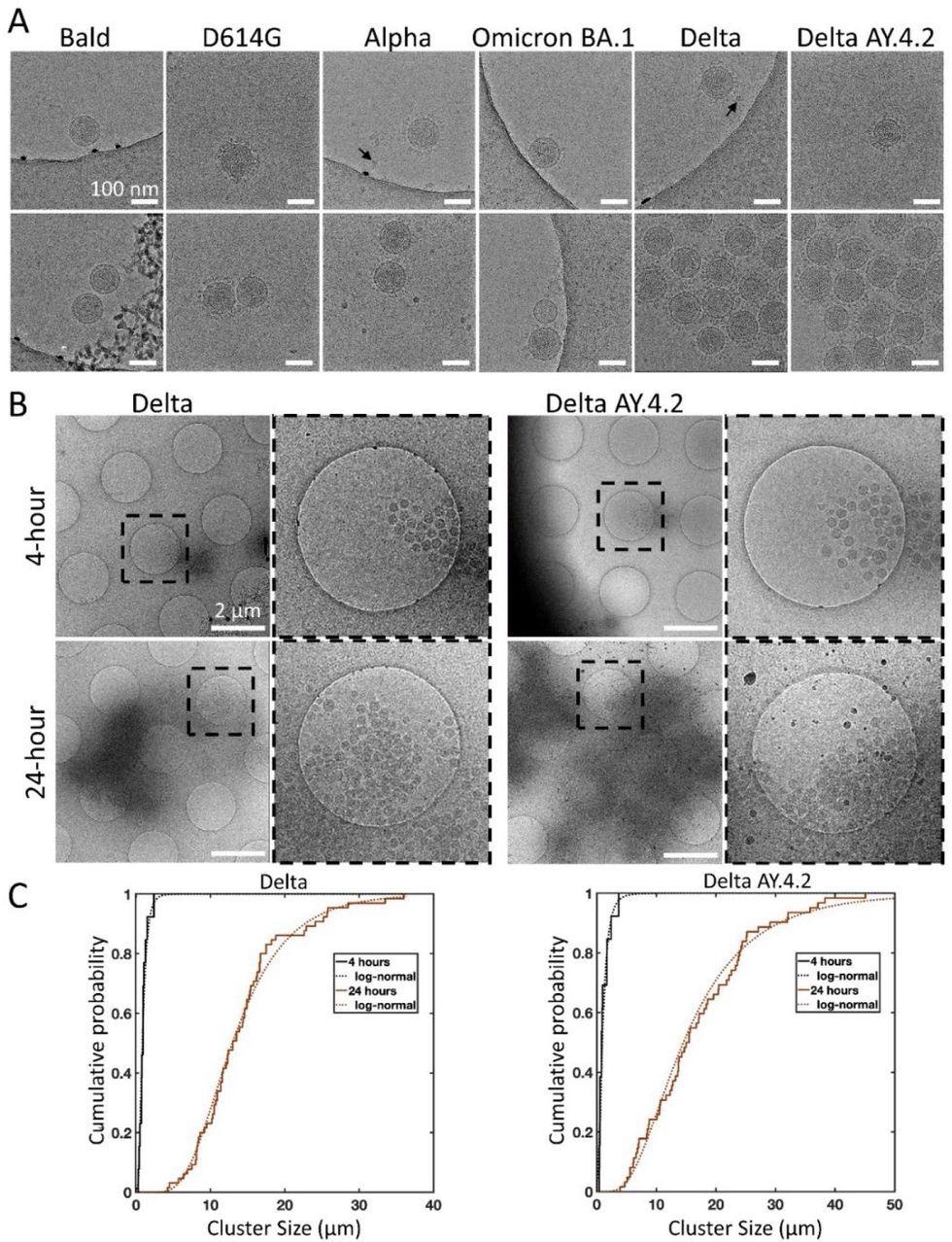
Cryo-electron microscopy of spike variant PPs. (A) Examples of singles (top panel), doubles, or aggregates (lower panel) of each variant PP. Spike variant is identified above. Black arrows indicate lipoproteins. The average PP envelope diameters for each variant are 118.6 (1.1), 118.5 (1.2), 119.6 (0.9), 120.1 (1.3), 115.8 (0.8), 118.4 (0.6) nm for Bald, D614G, Alpha, Omicron BA.1, Delta, and Delta AY.4.2, respectively (mean (SEM), n = 9, 33, 40, 18, 65, and 98. (B) Low magnification overviews of Delta and Delta AY.4.2 PP aggregates prepared 4 or 24 hours post-harvest. Dashed box shows the zoomed-in area. (C) The log-normal cumulative distributions for Delta and Delta AY.4.2 clearly show aggregation over time for both variants. At 4 and 24 hours, Delta aggregate sizes (longest axis) range from 0.28 – 2.39 and 4.20 – 36.0 μm, with log-normal parameters mu (sigma) −0.18 (0.56) and 2.56 (0.44) corresponding to means of 0.97 and 14.25 μm, respectively. There is no evidence for significant differences in aggregate sizes between Delta and Delta AY.4.2 for both 4 and 24 hours.
Conclusions
Overall, the study findings depicted a serendipitous discovery of substantial clustering of retrovirus PPs expressing the S protein of the SARS-CoV-2 Delta variant. By contrast, no aggregation was observed with the PPs consisting of the S proteins from the other three SARS-CoV-2 variants (Alpha, Omicron, and D614G mutant). Viral clustering could improve fitness by sheltering virions from environmental threats and facilitating collective infection. The initial viral infection could be favored by a collective infection in some circumstances. Moreover, the number and size of virions per cluster were significant in enhancing infectivity.
The distinct feature of the Delta S to cluster PPs might explain the faster SARS-CoV-2 infection by Delta PPs. In addition, S-mediated aggregation may also be a component of the molecular process by which Delta achieves greater transmissibility and rapid infection with significant viral loads.
The persistence of Delta PP aggregation over time suggests that the underlying factor for Delta PP clustering could be the S-S tip interactions. Further, this inference indicates an immune system-recognized adhesivity of the Delta surface, altering the balance of the host antiviral response to inflammation.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.