The Centers for Disease Control and Prevention (CDC), on November 29, 2021, advised that all individuals aged 18 years or older should receive a third mRNA-based SARS-CoV-2 vaccine booster dose at least six months following their primary two-dose regimen. However, following the emergence of the SARS-CoV-2 Omicron variant in the United States (US) on December 1, 2021, the advice for the six-month gap was reduced to at least five months on January 4 and 7, 2022, for the Pfizer-BioNTech and Moderna coronavirus disease 2019 (COVID-19) vaccines, respectively.
Compared to the SARS-CoV-2 Delta infections, the efficacy of two mRNA vaccine doses in preventing hospitalization or symptomatic illness due to Omicron infections was lower, with enhanced protection against both the viral variants after receiving the third dose. However, studies comparing the vaccine effectiveness (VE) of the second and third doses against symptomatic SARS-CoV-2 infection in outpatient settings during the Omicron and Delta variants’ predominant periods are scarce. This is particularly true for SARS-CoV-2 patients identified via active surveillance, wherein all enrolled subjects with COVID-19-like illness (CLI) are SARS-CoV-2-tested.
About the study
In the present study, the researchers assessed the SARS-CoV-2 Omicron and Delta variant-specific efficacy of the second and third doses of the COVID-19 mRNA-based vaccines in adults against symptomatic SARS-CoV-2 illness across the US outpatient settings. In addition, the scientists used viral sequencing data to identify the periods of Omicron and Delta predominance.
The current study was conducted under the US Flu VE Network (US Flu VE Network), consisting of health systems from seven US states. Between October 1, 2021, and February 12, 2022, researchers consented and recruited 3,847 eligible subjects who experienced cough, fever, or loss of smell or taste and accessed outpatient health care or clinical COVID-19 testing within ten days of disease onset. Employing the test-negative approach, the team compared the chances of receiving two or three mRNA SARS-CoV-2 vaccine doses among COVID-19 cases and controls through logistic regression. The regression models were controlled for research location, onset week, age, and previous COVID-19 history. Further, VE was estimated as (1-adjusted odds ratio) x 100%.
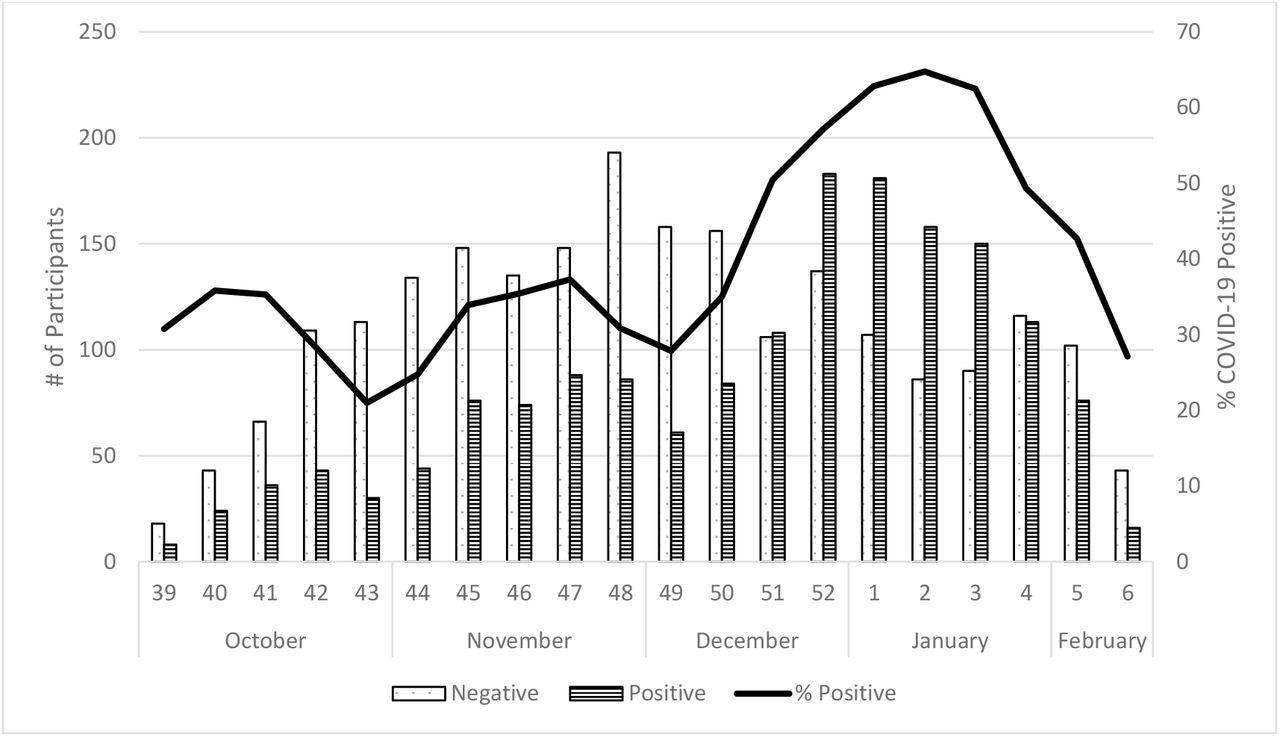
Number and Percent of SARS-CoV-2 Positive Enrolled Participants by Onset Week, US Flu VE Network, October 2021 – February 2022
Findings
The study results depicted that among the 3,847 eligible individuals575 out of 1775 subjects were COVID-19-positive during the SARS-CoV-2 Delta predominant phase, and 1,006 out of 1,794 subjects were SARS-CoV-2-positive during the Omicron predominant timeframe. The disease onset dates of the remaining 278 subjects fell between the established Omicron and Delta predominance periods. SARS-CoV-2 positivity surpassed 50% during the third week of December and peaked at 64% during the second week of January.
Subjects who self-reported fever or a high-risk exposure were more likely to test SARS-CoV-2-positive during the study. Participants who were ≥65 years old and belonged to the White/other races non-Hispanics were also more likely to receive a third COVID-19 vaccine dose than the Black Hispanic/non-Hispanic who self-reported a chronic health condition and did not self-report a fever. The median time between receiving a second dose and the onset of COVID-19 was 225 days among the two-dose vaccine recipients. Meanwhile, the average time between receiving the third dose and disease onset was 53 days.
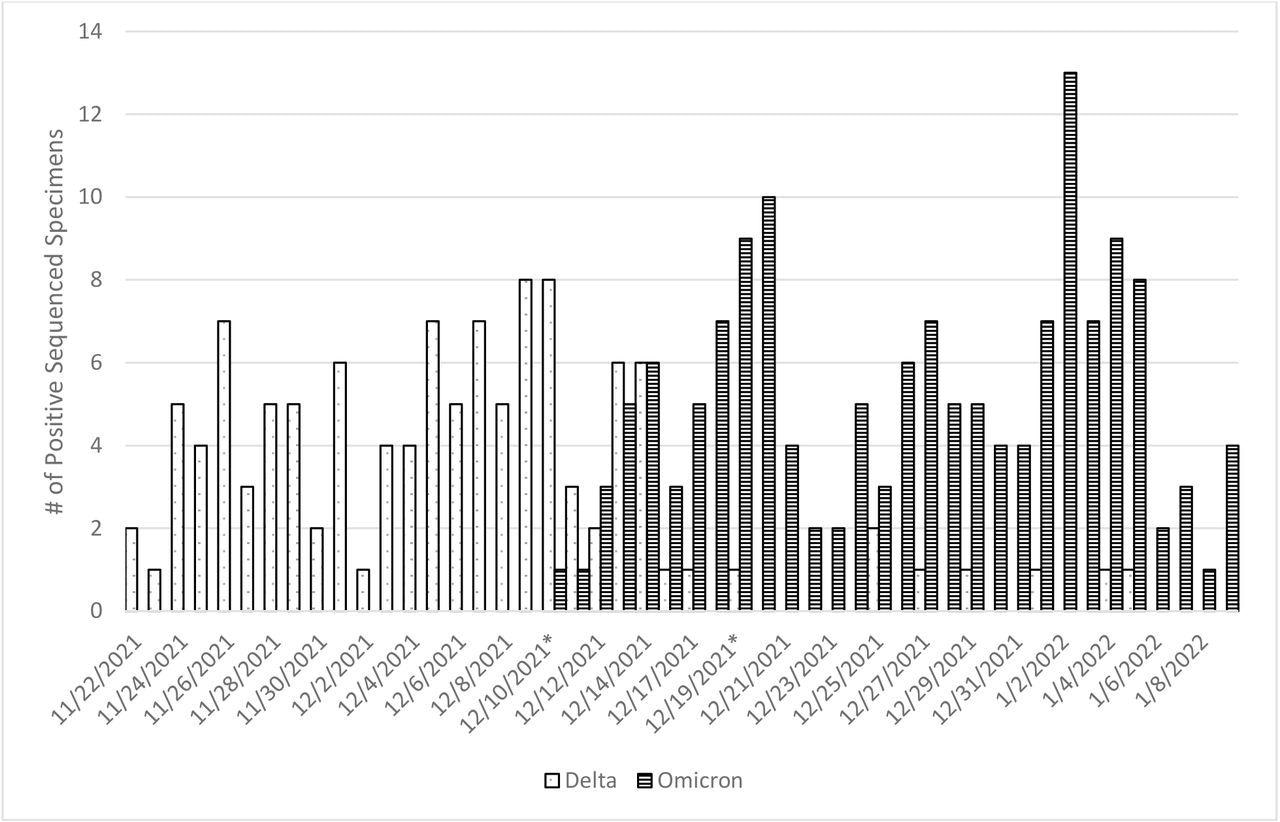
SARS-CoV-2 Variant Virus Distribution among Subset of Symptomatic SARS-CoV-2 Positive Participants by Onset Date, November 2021 – January 2022 *Variant transition period in the US Flu VE Network: December 10 – December 19, 2021
Sequencing data from 272 US Flu VE Network subjects with illness onset dates between November 9, 2021, and January 9, 2022, indicated that Delta constituted 45% of the sequenced specimens. On December 10, 2021, Omicron was first discovered in the network and became the predominant SARS-CoV-2 strain by December 15, 2021, with only a few Delta sequences detected in early January 2022.
Adjusted VE against symptomatic COVID-19 in the outpatient settings during the Delta period for mRNA vaccine two-dose and three-dose recipients were 63% and 96%, respectively. The corresponding adjusted VE against symptomatic COVID-19 during the Omicron period was 21% and 62%, respectively. Further, in the Delta-driven pandemic wave, VE among subjects who received the second dose on 14 to 149 days and ≥150 days before COVID-19 onset was 89% and 58%, respectively. The corresponding VE during the Omicron wave were 45% and 11%, respectively. Notably, the three-dose VE estimates were unaffected by the exclusion of 35 three-dose recipients who received the third dose <150 days after the second dose.
Furthermore, self-reported factors such as high-risk exposure status, CLI, previous laboratory-confirmed COVID-19, and presence of a chronic health condition did not alter the three-dose VE during the Delta predominant period. On the contrary, these determinants lowered three-dose VE in the Omicron dominancy.
Conclusions
The study findings showed that during the SARS-CoV-2 Delta predominant phase, VE against symptomatic COVID-19 in outpatient settings was 63% among the two-dose SARS-CoV-2 mRNA-based vaccinees and 96% for three-dose vaccinees. However, the corresponding VE was 21% and 62% during the Omicron predominance period, respectively. Although the three-dose VE among the subjects was reduced during the Omicron-driven COVID-19 wave than during Delta, the third mRNA dose imparted significant protection against symptomatic SARS-CoV-2 infection in both the Omicron and Delta predominant periods in outpatient settings.
Overall, the current study adds to the existing evidence of the efficacy of a third mRNA vaccine dose against laboratory-confirmed COVID-19 in people seeking outpatient treatment and clinical testing for CLI symptoms. The authors state that among the adult subjects, three doses of mRNA-based SARS-CoV-2 vaccines offered significant protection against symptomatic COVID-19 in outpatient settings during the Omicron-driven SARS-CoV-2 wave in the US. These data backed up the advice for the third dose of the COVID-19 mRNA vaccines by the CDC. Moreover, the study findings highlighted the requirement of COVID-19 booster vaccine doses or updates in vaccine formulations to elicit immunity against future SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Effectiveness of 2 and 3 mRNA COVID-19 Vaccines Doses against Omicron and Delta-Related Outpatient Illness among Adults, October 2021 – February 2022; Sara Kim, Jessie Chung, Keipp Talbot, Carlos G Grijalva, Karen Wernli, Erika Kiniry, Emily Toth Martin, Arnold Monto, Edward Belongia, Huong Q McLean, Manjusha Gaglani, Mufaddal Mamawala, Mary Patricia Nowalk, Krissy Moehling Geffel, Sara Tartof, Ana Florea, Justin S Lee, Mark W Tenforde, Manish Patel, Brendan Flannery, medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.04.06.22273535, https://www.medrxiv.org/content/10.1101/2022.04.06.22273535v1
- Peer reviewed and published scientific report.
Kim, Sara S., Jessie R. Chung, H. Keipp Talbot, Carlos G. Grijalva, Karen J. Wernli, Erika Kiniry, Emily T. Martin, et al. 2022. “Effectiveness of Two and Three MRNA COVID-19 Vaccine Doses against Omicron- and Delta-Related Outpatient Illness among Adults, October 2021–February 2022.” Influenza and Other Respiratory Viruses 16 (6): 975–85. https://doi.org/10.1111/irv.13029. https://onlinelibrary.wiley.com/doi/10.1111/irv.13029.