The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China late December 2019 and quickly spread throughout the world to ultimately cause the ongoing coronavirus disease 2019 (COVID-19) pandemic. More than two years later, the pathophysiology of COVID-19 remains poorly understood.
Study: The Spike Protein Of SARS-Cov-2 Impairs Lipid Metabolism and Increases Susceptibility to Lipotoxicity: Implication tor A Role of Nrf2. Image Credit: Skif_Nomad / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
SARS-CoV-2 is primarily known for its spike glycoprotein antigens that mediate host cell attachment and entry; however, this virus also produces multiple alterations in the metabolic pathways of the host cells. Thus, people with diabetes, metabolic syndrome, high cholesterol levels, obesity, and hypertension are at a higher risk of severe COVID-19 outcomes as compared to others.
Some evidence indicates that COVID-19 is associated with cardiovascular complications like myocardial injury and heart failure, which could be exacerbated in the presence of obesity. The authors of the present study previously reported a reduction in total and component lipoproteins in COVID-19 patients which, in turn, was associated with severe disease and fatal outcomes.
Lipids are essential to the viral life cycle in some viruses, and lipid rafts have been implicated in the replication of SARS viruses. Thus, the current study describes the effects of the viral spike protein on host lipid synthesis, turnover, and catabolism.
Study findings
In contrast to controls, the spike protein was associated with impaired lipid metabolism and autophagic processes in the host cell. As a result, host cells were more readily affected by lipotoxicity, which was likely due to pathways triggered by the activation of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated ferroptosis.
In cell lines expressing the spike protein, there was a greater accumulation of lipids when compared to control cells, particularly on the cell membrane. In close association with this change, which signals impaired lipid metabolism, a number of enzymes and lipid-associated proteins were upregulated. Simultaneously, other markers of autophagy and ferroptosis also exhibited altered levels of expression.
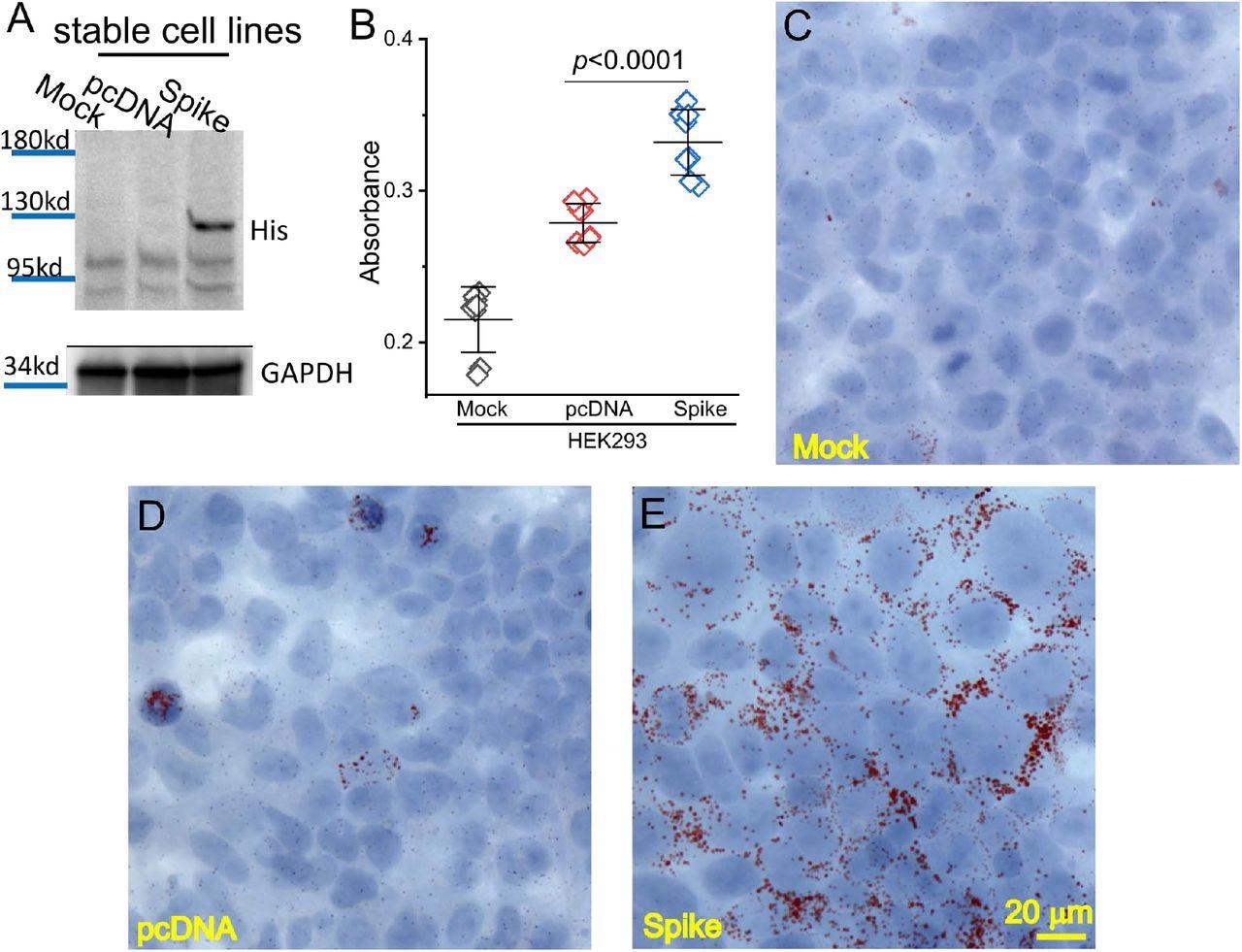
Spike protein caused lipid deposition in host cells. (A) Generation of Spike protein stable cell line. The expression of Spike protein was verified using an anti-His tag antibody in the Spike cells. (B) The quantification of Oil Red O staining in the mock, pcDNA, and Spike cells with measurement of an absorbance at 492 nm. (C-E) Histological images showing Oil Red O staining of lipid droplets in the mock (C), pcDNA (D), and Spike (E) cells. Nuclear components were counterstained by hematoxylin.
The overall impression is that the spike protein negatively affected these processes within the host cell. Moreover, with an overload of palmitic acid (PA) due to abnormal lipid processing within the cell, apoptosis was induced, thus indicating the toxic effects of the lipids. While this effect was observed in all cell lines where this fatty acid accumulated, the level of severity was enhanced in spike-bearing cells.
The effects of such an overload were dependent on both the duration of exposure and the level of PA in the cell. These cells also caused changes in the expression of some genes in response to the lipotoxicity of PA accumulation, within spike-expressing cells but not controls. In this situation, the levels of SRB1, ATG7, PTGS2, LC3 I/II ratio, and Fth1 were significantly higher as compared to controls.
In obesity, the cardiac muscle cells show impaired autophagy accompanied by ferroptosis that is mediated by Nrf2, the latter being the key to the changes in transcription seen in ferroptosis. Treatment with the Nrf2 inhibitor trigonelline (TRG), the ferroptosis inhibitor ferrostatin, and the PI3K inhibitor Wortmannin reduced the toxic effects of the lipid accumulation within the cells that occurred in response to the rise in PA that was enhanced in the presence of the spike protein.
Similar findings were obtained in response to the same process within a cell line that simulated the cardiomyocyte cell line. These observations indicate the role of the spike protein in exaggerated toxicity caused by the accumulation of PA.
Implications
The study findings demonstrate that the SARS-CoV-2 spike protein has a direct role in exacerbating the toxicity of lipid accumulation within the cell as a result of the disruption of many lipid pathways in response to the viral infection. This provides some insight into why obese patients are at a greater risk of severe COVID-19 as compared to those with normal body weight.
Inhibitors of Nrf2, PI3K, and ferroptosis were found to reduce this toxic effect, which was observed in the form of spike-induced necrosis. Interestingly, an alkaloid called TRG, which is found at high levels in coffee, is also an effective inhibitor of lipotoxicity. This represents “a potential and feasible preventive strategy to mitigate COVID-19-associated cardiometabolic pathologies associated with obesity.”
PI3K is key to the autophagy of infected cells during endocytosis and part of the growth factor receptor pathways that drive the phosphorylation of viral proteins following SARS-CoV-2 infection. Therefore, PI3K is a suitable target for drugs that counter or prevent COVID-19.
The viral spike protein can disrupt the expression of several PI3Ks in the host cells, thereby impairing the normal defensive response to SARS-CoV-2. This may be achieved by upregulating the formation of autophagosomes while reducing the fusion of autophagosomes with lysosomes. Autophagosomes subsequently accumulate within the cell, producing lipotoxicity.
Since Wortmannin shows the ability to oppose this exaggerated lipotoxicity, this agent, as well as other PI3K inhibitors, could be helpful in treating COVID-19 patients with high serum lipid levels.
Nrf2 is a regulator of over a thousand genes that participate in an array of metabolic and homeostatic pathways, such as detoxification, protein degradation, antioxidative defense, as well as iron and lipid metabolism. This broad functional capability of Nrf2 extends both to the adaptive capacity of the heart muscle and enhances maladaptive cardiac phenomena when the normal occurrence of myocardial autophagy is inhibited, as in chronic type 1 diabetes and obesity. This results in dysfunction of the heart musculature and abnormal remodeling.
The increase in Nrf2 expression in response to expression of the SARS-CoV-2 spike protein was mediated by PA accumulation and the resulting necrosis was suppressed by an Nrf2 inhibitor. Further research will be necessary to elucidate the exact underlying mechanisms of lipid metabolism dysregulation and determine how impaired Nrf2 signaling causes host cardiac cells in obese COVID-19 patients to experience ferroptosis induced by lipotoxicity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Nguyen, V., Zhang, Y., Gao, C., et al. (2022). The Spike Protein Of SARS-Cov-2 Impairs Lipid Metabolism and Increases Susceptibility to Lipotoxicity: Implication tor A Role of Nrf2. bioRxiv. doi:10.1101/2022.04.19.488806. https://www.biorxiv.org/content/10.1101/2022.04.19.488806v1.
- Peer reviewed and published scientific report.
Nguyen, Vi, Yuping Zhang, Chao Gao, Xiaoling Cao, Yan Tian, Wayne Carver, Hippokratis Kiaris, Taixing Cui, and Wenbin Tan. 2022. “The Spike Protein of SARS-CoV-2 Impairs Lipid Metabolism and Increases Susceptibility to Lipotoxicity: Implication for a Role of Nrf2.” Cells 11 (12): 1916. https://doi.org/10.3390/cells11121916. https://www.mdpi.com/2073-4409/11/12/1916.