The BCG vaccine has been effectively used to vaccinate over 130 million children in almost 154 countries against tuberculosis (TB) every year. Various studies have also suggested the vaccine's usefulness against other diseases, including viral infections.
 Study: BCG vaccination of Diversity Outbred mice induces cross-reactive antibodies to SARS-CoV-2 spike protein. Image Credit: Garna Zarina / Shutterstock
Study: BCG vaccination of Diversity Outbred mice induces cross-reactive antibodies to SARS-CoV-2 spike protein. Image Credit: Garna Zarina / Shutterstock

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
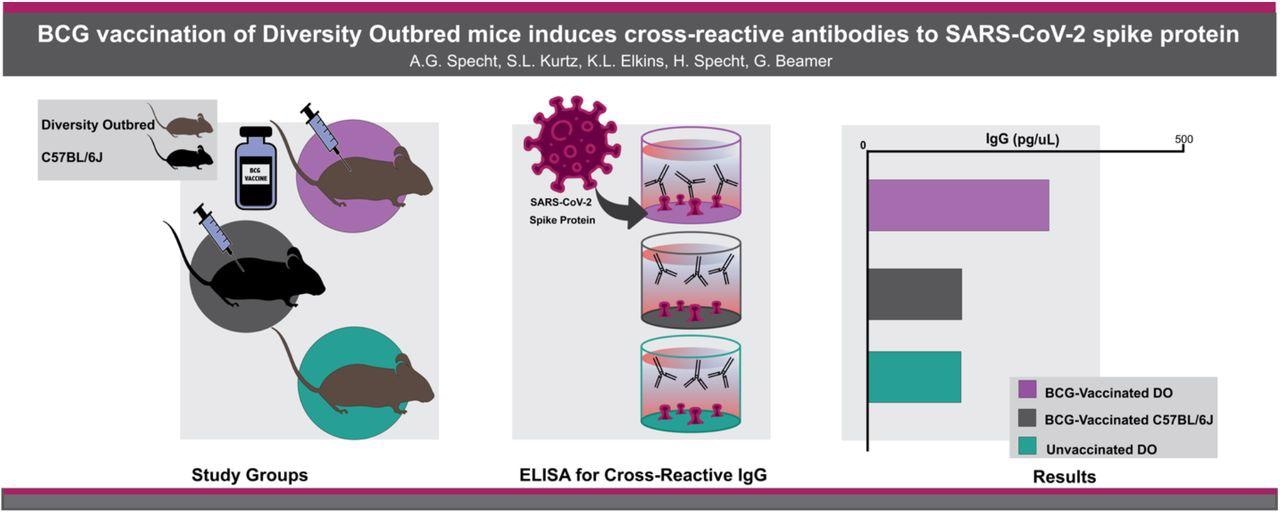
The present study measured the cross-reactivity of antibodies (Abs) induced by the BCG vaccine against the SARS-CoV-2 S protein in distinct mouse strains. The team also compared the amount of SARS-CoV-2 Abs in serological samples of BCG-vaccinated, outbred Diversity Outbred (DO) and inbred C57BL/6J mice with the serum samples of unvaccinated DO mice.
The researchers predicted the potential cross-reactive Ab binding sites via in silico comparison of the Ab epitopes found on the SARS-CoV-2 S protein and the BCG proteome. Subsequently, the linear epitopes with more than five amino acids in the receptor-binding domain (RBD) were searched in a database of BCG Pasteur proteins. The subsequent homologous BCG proteins were then characterized according to their location on or in the BCG strain Mycobacterium bovis.
The predicted Ab binding epitopes on the S protein were selected using the Bepipred linear epitope prediction 2.0. The selected epitopes were subsequently aligned with the BCG proteome using the basic local alignment search tool (BLAST). Moreover, the team conducted an enzyme-linked immunosorbent assay (ELISA) to detect cross-reactive immunoglobulin G (IgG) Abs in the collected mouse sera. An independent assay control was used to evaluate the quality of Ab detection and colorimetric reaction.
Serum samples were collected from the vaccinated C57BL/6J mice, the vaccinated DO mice, and the unvaccinated DO mice. The team compared the serological levels of IgG-binding SARS-CoV-2 recombinant-S protein between BCG-vaccinated DO mice, BCG-vaccinated C57BL/6J mice, and unvaccinated DO mice. Furthermore, the serum samples from C57BL/6J mice vaccinated intravenously and intradermally were compared with that from BCG-vaccinated and unvaccinated DO mice.
Results
The study results showed that 25 potential epitopes having more than five amino acids were found on the SARS-CoV-2 S protein. Moreover, the BLAST search showed 186 peptides with seven amino acids or more on the BCG proteome, all of which matched the description of a BCG protein. Among these, 59.7% had known cellular composition, with most of the proteins being cytoplasmic. However, five of the proteins were located on the cell's surface. Overall, this indicated that the SARS-CoV-2 S protein and BCG surface proteins shared several amino acid sequences.
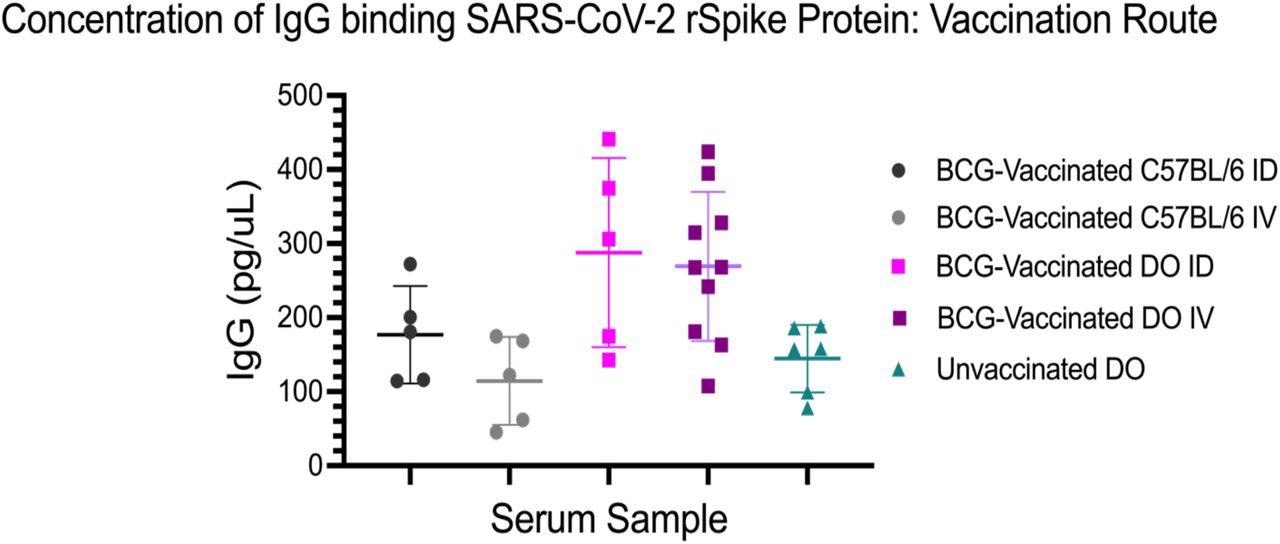
Concentration of IgG binding SARS-CoV-2 rSpike protein by vaccination route and mouse strain. Each data point is the mean concentration based on technical triplicates at 1:500 dilution. C57BL/6J mice vaccinated intradermally (ID) (n=5) and intravenously (IV) (n=5) were compared with DO (J:DO) mice vaccinated ID (n=5), IV (n=10) and unvaccinated (n=5). The Student’s t-test was used to determine if the means of two groups are significantly different. IgG concentrations for BCG-vaccinated DO mouse sera (both ID and IV) were significantly greater than both BCG-vaccinated C57BL/6J (both ID and IV) and unvaccinated DO mouse sera.
The team observed that 67% of the BCG-vaccinated DO mice generated more SARS-CoV-2 S protein reactive Abs as compared to the BCG-vaccinated C57BL/6J mice and the unvaccinated DO mice. Moreover, the mean concentration of IgG reactive to the S glycoprotein was 292 pg/mL in the BCG-vaccinated DO mice, 146 pg/mL in the vaccinated DO mice and 144 pg/mL in the BCG-vaccinated C57BL/6J mice. Thus, no significant difference was found in the IgG concentrations of the unvaccinated DO and the BCG-vaccinated C57BL/6J mice. The substantial difference in the IgG concentrations in the BCG-vaccinated C57BL/6J mice potentially indicated assay sensitivity and possible genetic differences that influence humoral immune responses.
Furthermore, the analysis of the vaccination route showed that the IgG concentration in the intravenously and intradermally vaccinated C57BL/6J mice was 177 pg/mL and 115 pg/mL, and DO mice were 288 pg/mL and 269 pg/mL, respectively. Moreover, the route of vaccination did not significantly impact the concentration of IgG reactive to SARS-CoV-2 recombinant S protein for either of the mice strains.
To summarize, the study findings showed that a subset of the BCG-vaccinated DO mice generated a higher concentration of reactive IgG Abs reactive to the SARS-CoV-2 S protein as compared to the unvaccinated DO mice and the BCG-vaccinated C57BL/6J mice.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.