CgA, a 439-residue-long protein, and its 76-residue long polypeptide fragment vasostatin I (VS-I) are released into the blood from the endocrine and neuroendocrine cells. CgA and VS-I control stress responses in the body. Prior reports demonstrated that elevated CgA levels were observed in patients with heart failure, neuroendocrine tumors, arterial hypertension (HTN), diabetes mellitus (DM), rheumatoid arthritis, renal failure, and inflammatory bowel diseases and were associated with mortality risk.
Further, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection manifests in varying levels of severity. In certain COVID-19 patients, a weaker nonspecific immune response fails to stop virus replication, leading to metabolic derangements, multiorgan failure, and acute respiratory distress syndrome. Existing studies have indicated severe COVID-19 was linked to pre-existing comorbidities (HTN, respiratory diseases, DM, and cardiovascular diseases) and age. They also noted that COVID-19-related neurological, cardiovascular, vascular, and renal complications were linked to a higher risk of death. Nevertheless, CgA and VS-I's role in COVID-19 has yet to be determined.
About the study
The present work aimed to determine if the release of CgA and VS-I in the circulation is part of the initial host response in SARS-CoV-2 infection and if these compounds, detected at the start of COVID-19, could portend adverse outcomes. Therefore, the investigators measured the plasma concentrations of CgA and VS-I in 190 COVID-19 patients upon hospital admission between March and May 2020. Study controls were 40 healthy volunteers who were sex and age-matched with the subjects.
The Spearman correlation test or the Mann-Whitney U test was used to analyze the connection between CgA and VS-I levels with SARS-CoV-2 severity, patient demographics, and comorbidities. The effect of CgA and VS-I concentrations on COVID-19-linked in-hospital death was investigated using Kaplan Meier survival curves and Cox regression analysis.
Findings
The study results showed that COVID-19 patients had higher median CgA and VS-I levels than healthy controls, i.e., CgA: 0.558 nM versus 0.368 nM; VS-I: 0.357 nM against 0.144 nM, respectively. CgA concentrations were considerably higher in 47 patients who died (median 0.948 nM) than in 143 survivors (median 0.507 nM). The discovery that CgA accumulates in COVID-19 patients, with a preference for those who die, provides a tool for disentangling the immune/neuroendocrine relationship in the SARS-CoV-2 infection host response.
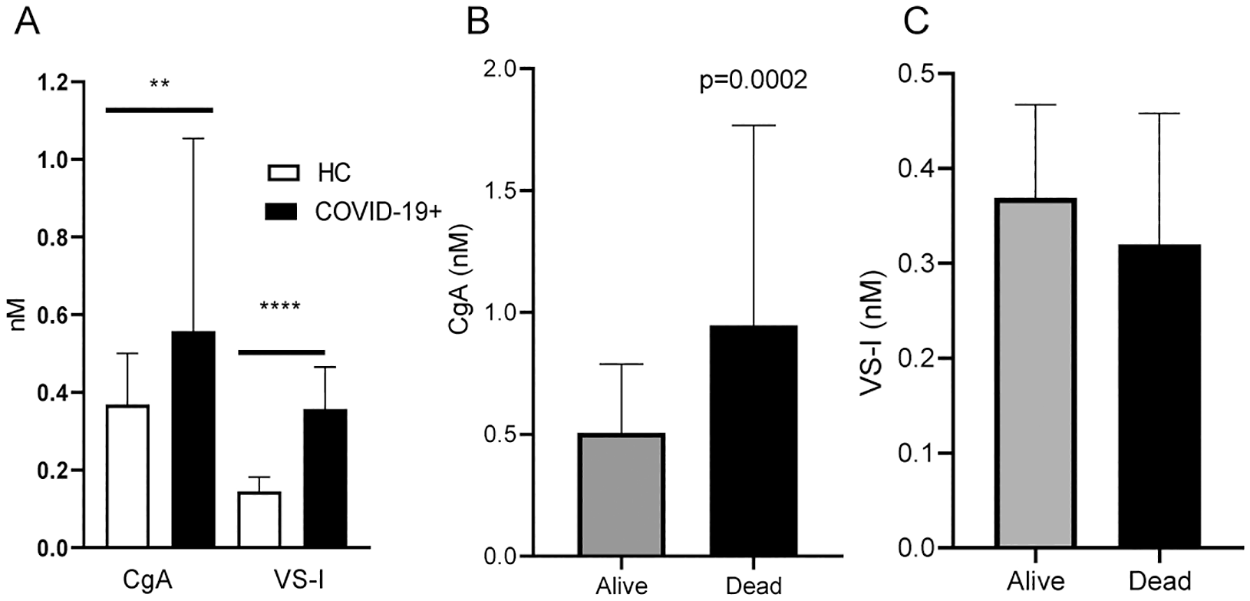
Panel A: CgA and VS-I plasma levels in age- and sex-matched healthy controls (HC) and COVID-19 patients at hospital admission. Panel B: CgA plasma levels in COVID-19 patients with favorable outcome (Alive) or who died (Dead). Panel C: VS-I in Alive or Dead patients. ** p<0.001.
The investigators discovered a link between plasma CgA concentrations and age and that CgA levels were high in COVID-19 patients with comorbidities. When controlled for the number of comorbidities, age, the degree of respiratory insufficiency, the time from SARS-CoV-2 symptom start to sampling, and C-reactive protein levels, CgA levels were found to be independent predictors of in-hospital death (hazard ratio 1.28). According to Kaplan Meier curves, COVID-19 patients with CgA concentrations over 0.558 nM had a considerably higher death rate.
Interestingly, VS-I plasma concentrations in SARS-CoV-2 patients were similar in non-survivors and survivors, although they were elevated than in healthy controls. In addition, the authors found that the amount of CgA released into the blood predicted clinical outcomes in COVID-19 patients. In contrast, in individuals with the severe SARS-CoV-2 outcome, the proteolytic processing of CgA that yields VS-I was not altered, implying that the molecular machinery involved in VS-I generation was similarly controlled in COVID-19 patients irrespective of disease development.
Further, the results indicated that the CgA concentrations were linked with systemic inflammation and hypoxia. However, the authors failed to discover the mechanism and origin of CgA generation in SARS-CoV-2 patients.
Conclusions
According to the investigators, this was the first study on CgA and VS-1 concentrations in SARS-CoV-2 infection that demonstrated the link between these molecules and clinical outcomes of COVID-19, as measured by in-hospital death.
The study findings revealed that plasma VS-I and CgA levels rise in SARS-CoV-2 patients, indicating a neuroendocrine stimulation in these patients. The present data then implied that CgA and not VS-I could be an early independent predictor of death. CgA plasma concentration upon COVID-19-related hospital admission might thus serve as a tool for identifying patients who are at a higher risk of adverse disease progression and, as a result, require more intensive therapy. The investigators further stated that more research is needed to determine the role of CgA and the feasibility of using the signal to early stratify SARS-CoV-2 patients depending on their likelihood of negative consequences.