Many patients do not completely recover from COVID 2019 (COVID-19) and subsequently suffer from Long COVID or post-acute sequelae of SARS-CoV-2 (PASC). Several factors, including infecting SARS-CoV-2 variant, vaccine status of the individual contracting the disease, and the COVID-19 treatment approach, contribute to the development of this health condition.
Nevertheless, there is an urgent need to acknowledge the need for preventing and reversing this potentially serious condition, which is only possible with a better understanding of the pathophysiology of PACS symptoms.
In the present study, researchers examined three patients who received nirmatrelvir/ritonavir antivirals for COVID-19 treatment.
 Study: Effect of oral nirmatrelvir on Long COVID symptoms: a case series. Image Credit: Donkeyworx / Shutterstock
Study: Effect of oral nirmatrelvir on Long COVID symptoms: a case series. Image Credit: Donkeyworx / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Patient 1
A 48-year-old man developed fever, headache, and pharyngitis in the Spring of 2022. Post receiving a positive reverse transcription-polymerase chain reaction (RT-PCR) test for SARS-CoV-2, a five-day therapy of nirmatrelvir/ritonavir was started.
Since he started antiviral therapy within 24 hours of the appearance of symptoms, his symptoms quickly resolved. Notably, this patient had a medical history of Bechet’s disease on colchicine. Moreover, he had received a three-dose regimen of a COVID-19 vaccine five months before contracting the disease.
Around four days later, he again experienced recurrent fatigue, chest pain, fever, and brain fog but did not get re-tested for COVID-19. His symptoms persisted for more than 30 days following the onset of symptoms.
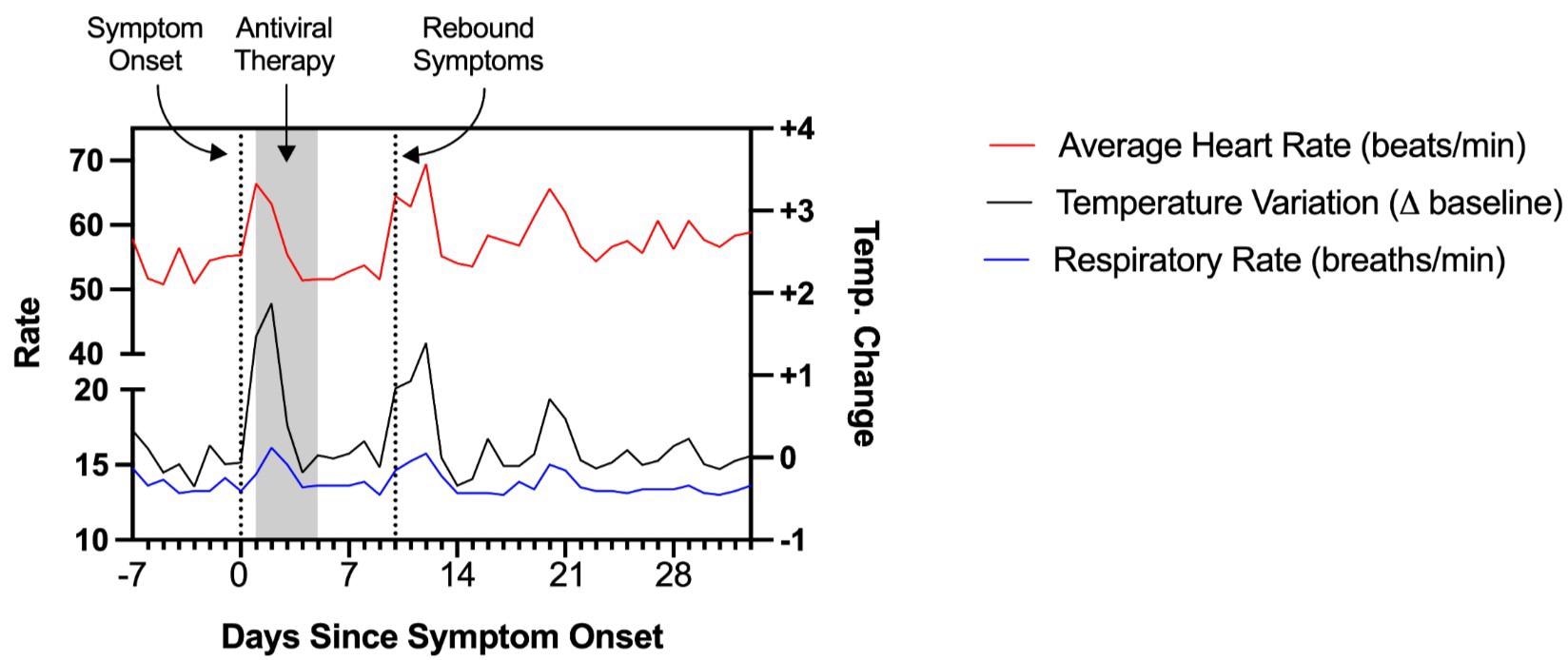 Physiologic measurements as recorded by a personal fitness device used by Patient 1, showing rebound tachycardia, tachypnea, and elevated body temperature coinciding with completion of antiviral therapy.
Physiologic measurements as recorded by a personal fitness device used by Patient 1, showing rebound tachycardia, tachypnea, and elevated body temperature coinciding with completion of antiviral therapy.
Patient 2
A 42-year-old man developed pharyngitis and rhinorrhea at the beginning of 2022. He suffered from myalgia, severe fatigue, and a rash on his groin, and his symptoms persisted for around 10 days. After 11 days, all his symptoms disappeared, and his rapid antigen test (RAT) turned SARS-CoV-2-negative. After approximately two weeks, the patient again experienced new symptoms, such as pain across his upper body bones. He also had more fatigue and lung soreness, i.e., increased awareness of breathing which persisted for around seven weeks following the initial symptom onset and worsened.
His health condition deteriorated to 40 to 50% of his pre-COVID-19 baseline health, and he rested throughout the day, unable to resume his routine life. During this time, the patient suffered a re-exposure to SARS-CoV-2 as his family tested SARS-CoV-2-positive on RATs. Within a few days post-re-exposure, he witnessed an improvement in his worsening symptoms.
A five-day oral antiviral therapy of nirmatrelvir/ritonavir was initiated. Although his symptoms did not resolve entirely, they continually improved, and he reported restoring his pre-COVID-19 health.
Patient 3
A 43-year-old woman developed pharyngitis and cough and tested COVID-19-positive on RAT five days later in the Spring of 2022. However, in the coming three weeks, she began to experience malaise and fatigue, concomitantly with myalgia and brain fog. Notably, she had no underlying health conditions and also received a three-dose regimen of a COVID-19 vaccine four months before contracting the disease.
Nirmatrelvir/ritonavir treatment was initiated 25 days after the initial symptoms and experienced respite from fatigue following the completion of therapy. Although she still had some shortness of breath and myalgias, she resumed her routine daily chores.
Discussion and conclusion
The clinical anecdotes presented in the study appear informative for researchers evaluating the pathophysiology governing the development of Long COVID.
Further, these case studies suggested that the SARS-CoV-2 burden during acute infection widow might be a key factor determining the development of Long COVID. Although early antiviral therapy might diminish the risk of developing severe disease, it may be insufficient to prevent the development of Long COVID.
Therefore, Patient 1, who received brief early antiviral therapy as per the emergency use authorization (EUA) criteria, experienced a recurrence of COVID-19 symptoms with associated physiologic changes as observed by a personal fitness device. Subsequently, he experienced worsening post-infection symptoms, which met the United States Centers for Disease Control (CDC) criteria for Long COVID.
Another hypothesis suggests that SARS-CoV-2 persists for several months in some individuals. Although not EUA approved, patients access antiviral therapy beyond the acute COVID-19 infection window.
Patients 2 and 3 had different timing of infection, i.e., Winter 2021–2022 vs. Spring 2022. Likewise, their timing of antiviral therapy from initial infection was different, i.e., greater than 60 days and less than 30 days, respectively. In their cases, persistent viral activity, especially in the tissues, could be responsible for persistent Long COVID symptoms. Therefore, it is crucial to assess the impact of antiviral therapy beyond the acute infection window of SARS-CoV-2 infection.
Another intriguing observation was the improvement in COVID-19 symptoms following SARS-CoV-2 re-exposure in the Case 2 patient. It raised the possibility of recalibration of a dysregulated immune response.
Overall, the current short case series suggested a complex interplay between antiviral therapy/antigen re-exposure and SARS-CoV-2 replication and the host immune response underlying the development of Long COVID.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Michael J. Peluso, Khamal Anglin, Matthew S. Durstenfeld, Jeffrey N. Martin, J. Daniel Kelly, Priscilla Y. Hsue, Timothy J. Henrich, Steven G. Deeks, Effect of oral nirmatrelvir on Long COVID symptoms: a case series, Research Square pre-print 2022, DOI: https://doi.org/10.21203/rs.3.rs-1617822/v1 https://www.researchsquare.com/article/rs-1617822/v1
- Peer reviewed and published scientific report.
Peluso, Michael J, Khamal Anglin, Matthew S Durstenfeld, Jeffrey N Martin, James J Kelly, Priscilla Y Hsue, Timothy J Henrich, and Steven G Deeks. 2022. “Effect of Oral Nirmatrelvir on Long COVID Symptoms: 4 Cases and Rationale for Systematic Studies.” Pathogens & Immunity 7 (1). https://doi.org/10.20411/pai.v7i1.518. https://www.paijournal.com/index.php/paijournal/article/view/518.