On March 11, 2020, the World Health Organization (WHO) declared a pandemic status to the coronavirus disease 2019 (COVID-19) outbreak caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that was first detected in Wuhan, China.
The pandemic continues, but vaccines are doing their part to stop the spread of the disease. However, the currently available vaccines only curtail the viral transmission and disease severity; they do not prevent the infection. Hence, new antiviral strategies are warranted for mitigating COVID-19 and for widespread viral control.
Convalescent human plasma was one of the initial therapies employed for controlling COVID-19 symptoms. Major challenges posed by this modality were quality control and standardization, along with optimal dosing and administration timepoints, apart from the risk of other infections.
Intravenous immunoglobulins (IVIG) treatment could overcome all these issues as they are sterile plasma collected from multiple donors. Yet, this method too relies on human material as the source. Therefore, the large-scale production of animal-derived antibodies is more feasible.
Ovine immunoglobulin G (IgG)-based products – which act as snake antivenoms, have also been used for treating infectious diseases like rabies. These polyclonal antibodies target multiple epitopes and exhibit numerous functions, such as – impeding viral attachment, viral aggregation and clearance from circulation, activation of phagocytosis, and complement system activation.
 Study: Development of a cost-effective ovine antibody-based therapy against SARS-CoV-2 infection and contribution of antibodies specific to the spike subunit proteins. Image Credit: extender_01 / Shutterstock
Study: Development of a cost-effective ovine antibody-based therapy against SARS-CoV-2 infection and contribution of antibodies specific to the spike subunit proteins. Image Credit: extender_01 / Shutterstock
The study
A recent study published in the journal Antiviral Research explored alternative sources of polyclonal antibodies against SARS-CoV-2, which would be more cost-effective, amendable to regulatory approval, and widespread use.
Here, the ovine polyclonal antibody-based candidate was examined to develop a cost-effective therapy targeting COVID-19.
For this study, SARS-CoV-2 spike (S) glycoprotein was generated in Chinese Hamster Ovary (CHO), inclusive of a His-tag. Cells from human embryonic kidneys (HEK293) were sensitized to produce SARS-CoV-2 subunits - S1 and S2 proteins, with a sheep Fc tag.
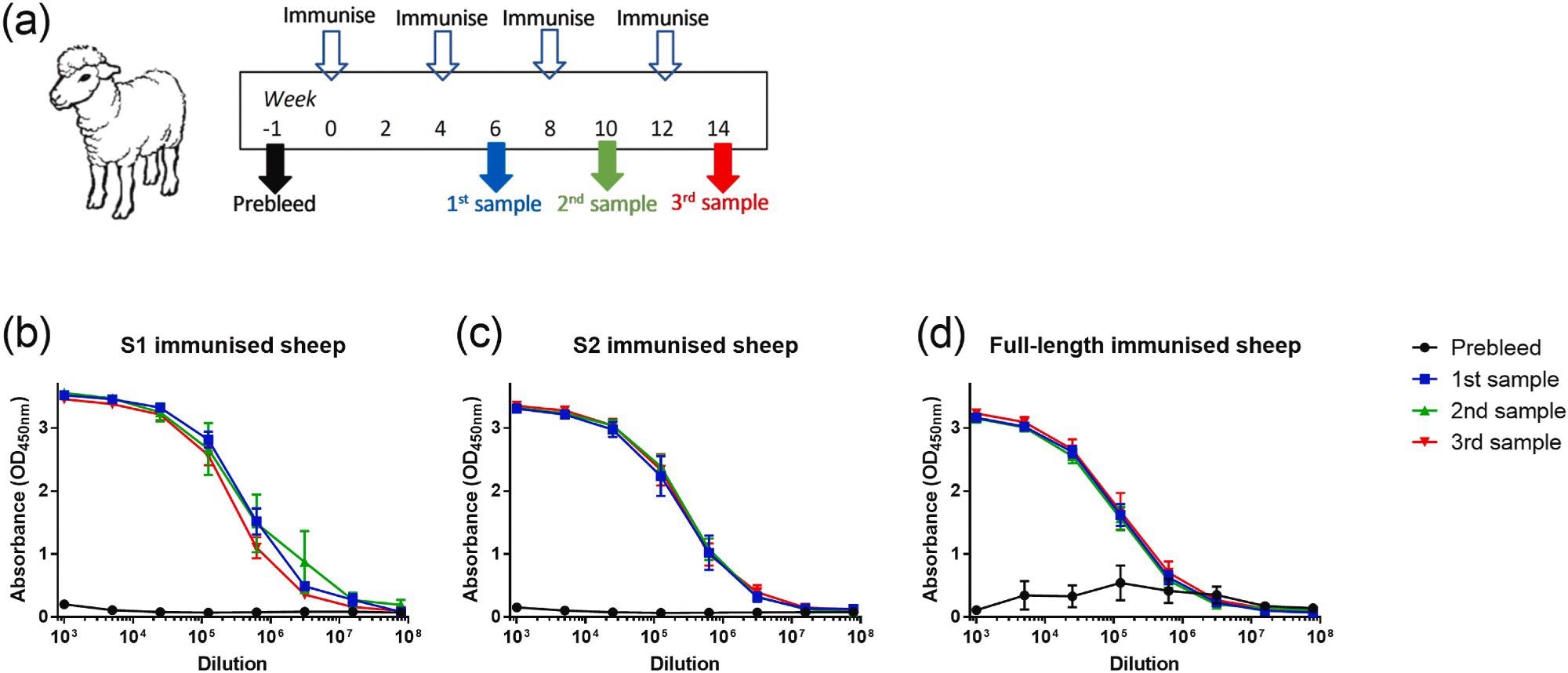 Binding of ovine sera to full-length recombinant SARS-CoV-2 spike protein after immunization with glycoprotein antigens. (a) Outline of study schedule. (b) Reactivity of S1-immunised sheep, n = 3. (c) Reactivity of S2-immunised sheep, n = 3. (d) Reactivity of full-length immunised sheep, n = 6. Lines show mean values with error bars denoting standard error.
Binding of ovine sera to full-length recombinant SARS-CoV-2 spike protein after immunization with glycoprotein antigens. (a) Outline of study schedule. (b) Reactivity of S1-immunised sheep, n = 3. (c) Reactivity of S2-immunised sheep, n = 3. (d) Reactivity of full-length immunised sheep, n = 6. Lines show mean values with error bars denoting standard error.
Results
Following two immunizations with recombinant SARS-CoV-2 proteins, sheep produced robust antibody responses. The antibody titers remained high after subsequent monthly immunizations.
Plasma was collected at all time points and pooled and processed to render stocks of purified IgG. Purified IgG was then tested alongside antibody concoctions that had been affinity purified.
After immunization with the full-length S protein and either the S1 or S2 subunit, antibodies were observed that recognized recombinant whole S protein. Antibodies produced by sheep who received S2 antibodies rendered lower S1-specific responses. Similarly, S1 antibodies produced low S2-specific responses.
Moreover, affinity-purified preparations showed higher binding than that of the purified IgG. The antibody generated in response to full-length S protein exhibited the highest neutralizing potential against the Wuhan-like strain (IC50 value - 49 ng/ml). Antibodies against S1 and S2 proteins also had neutralizing activity (IC50 values 1.071 and 0.428 μg/ml, respectively).
All preparations expressed strong neutralizing activity against both SARS-CoV2 strains; however, the Omicron BA.1 variant necessitated ∼10-fold higher antibody concentrations for an optimal effect.
All three antibody preparations elicited antibody-dependent complement deposition––which was relatively higher for sheep immunized with full-length S protein.
Additionally, an antibody-dependent neutrophil phagocytosis assay also disclosed that the full-length S antibodies had comparatively higher activity than S1-specific antibodies. However, the S2-specific antibodies showed lower opsonophagocytosis.
Furthermore, full-length S protein antibodies exhibited strong reactions to the receptor-binding domain (RBD) and S antigens from different variants of SARS-CoV-2, for instance –alpha, beta, and gamma. On the other hand, the S1-derived preparation was only active against the SARS-CoV-2 mutants, while the S2-derived antibodies had limited cross-reactivity.
Overall, the full-length S protein antibodies conferred a consistently higher level of interaction compared to the subunit antibody preparations, with the S2-derived antibodies granting inhibition levels of 50.7% and below.
The efficacy of antibodies in the prevention of COVID-19 was assessed. For this, a hamster was administered 2 mg antibody 24 hours before the challenge. The findings revealed that these hamsters lost less weight compared to the controls (administered 2 ml of affinity-purified antibodies diluted to a concentration of 1 mg/mL with phosphate-buffered saline). The groups that received full-length or S2 antibodies had no evidence of weight loss.
Of note, weight loss was comparatively more significant in all controls. In addition, the control animals had higher clinical scores.
When the concentration of circulating antibodies was evaluated, it was observed that one animal in the full-length S protein and S1 subunit groups had low amounts of antibodies. It was inferred that animals with low ovine-specific antibody experienced a higher severity of clinical disease.
Viral RNAs tested in pharyngeal swabs sustained similar measures amongst all animals who had been given antibodies compared to the controls. However, the eighth day recorded notably lower viral RNA levels in animals who received S2-specific antibodies.
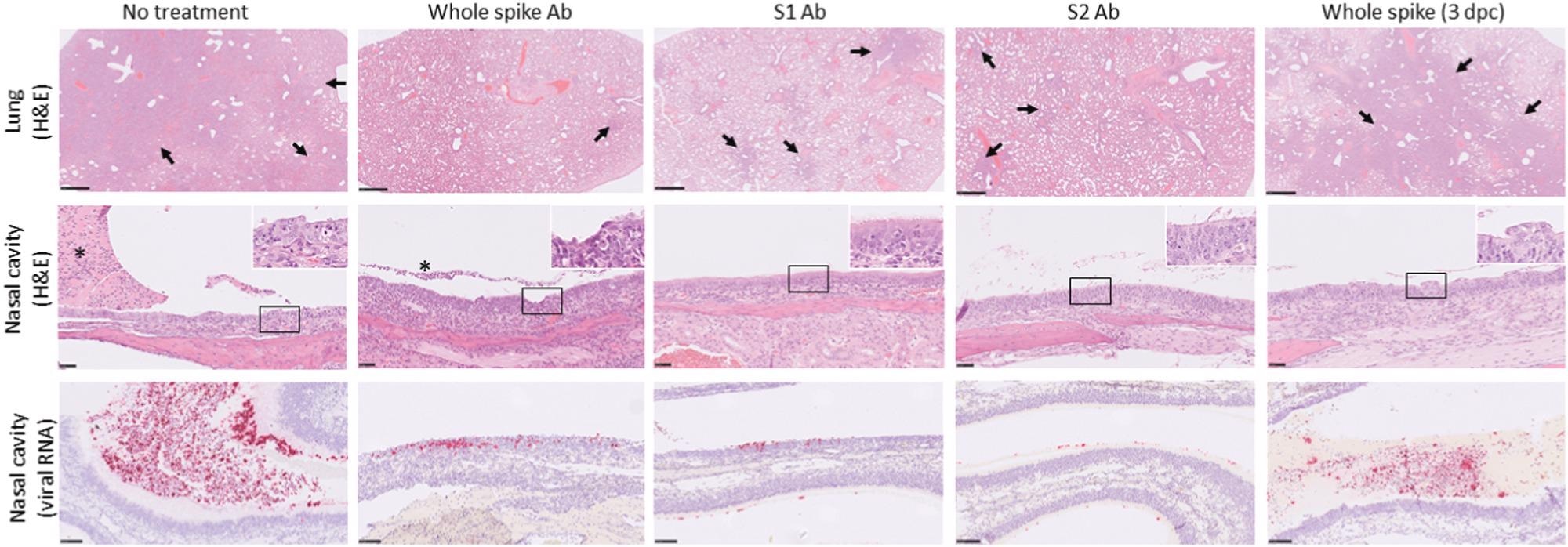 Representative microscopic images of lungs and nasal cavities of hamsters receiving ovine antibody preparations after challenge with SARS-CoV-2. Top row, lung-multifocal to patchy areas of pneumonic consolidation (arrows) (H&E); middle row, nasal cavity-inflammation and degeneration of the mucosa with variable luminal exudate (asterisks). Inset, higher power images of nasal epithelium (×800 magnification) (H&E); lower row, nasal cavity-staining for SARS-CoV-2 viral RNA in the mucosa and luminal exudate (in situ hybridization).
Representative microscopic images of lungs and nasal cavities of hamsters receiving ovine antibody preparations after challenge with SARS-CoV-2. Top row, lung-multifocal to patchy areas of pneumonic consolidation (arrows) (H&E); middle row, nasal cavity-inflammation and degeneration of the mucosa with variable luminal exudate (asterisks). Inset, higher power images of nasal epithelium (×800 magnification) (H&E); lower row, nasal cavity-staining for SARS-CoV-2 viral RNA in the mucosa and luminal exudate (in situ hybridization).
Viral RNA levels were lower in the lungs of animals who received antibody compounds before the challenge – this finding was significant in the S2-derived antibody group. SARS-CoV-2 characteristic lung lesions could be visualized in the left lung lobe and nasal cavity of all animals. The lung lesions were described as multifocal, coalescing broncho-interstitial pneumonia with regions of consolidation.
The nasal cavity contained exudates – fluid with inflammatory cells, and also exhibited degeneration and necrosis of respiratory and olfactory mucosa.
Animals who received full-length and S2-specific antibodies had less severe lung lesions. Meanwhile, the pneumonic change was reduced in the S2-subunit group. The findings illustrated lower lung pathology and histological scores in animals that received prior antibody treatment (before challenge) than those who received delayed antibody (post-challenge).
One risk imposed by antibodies against SARS-CoV-2 was addressed – that of antibody-dependent enhancement (ADE). ADE takes effect by enabling viral entry into the host cells via the human Fc receptor. Antibodies discussed in the present study are raised in sheep, and thus, eradicated ADE.
These findings may be of importance in mitigating the COVID-19 pandemic and preventing threats of future outbreaks.