Omicron (B.1.1529) has demonstrated higher infectivity compared to other SARS-CoV-2 variants. In addition, studies have reported lower Omicron neutralization by the existing COVID-19 vaccines. Despite this, it is not clear just how much protection the COVID-19 vaccine confers against Omicron infections.
 Study: Covid-19 vaccine effectiveness against general SARS-CoV-2 infection from the omicron variant: A retrospective cohort study. Image Credit: Claudio Bottoni
Study: Covid-19 vaccine effectiveness against general SARS-CoV-2 infection from the omicron variant: A retrospective cohort study. Image Credit: Claudio Bottoni

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, researchers evaluated the immune protection conferred by two- and three-dose regimens of the mRNA-1273 and BNT162b2 vaccines against Omicron infections among students and employees of the Clemson University in South Carolina (SC), United States of America (USA).
The study was conducted on 24,145 individuals, comprising 18-to-24-year-old university students and 18-to-64-year-old university employees, who were undergoing COVID-19 testing on a weekly basis between January 3 and January 31, 2022. Data on the participants’ vaccination status (vaccine manufacturer and first, second and third vaccination dates) were collected by the voluntary upload of vaccination cards via Clemson University’s COVID-19 Voluntary Vaccine Upload Tool. The weekly SARS-CoV-2 testing included polymerase chain reaction (PCR) tests on specimens such as saliva or nasal swabs or throat swabs.
The individuals were excluded if they were student-athletes, individuals who received a vaccine other than the mRNA-1273 or BNT162b2 vaccines, or individuals who received the second BNT162b2 dose within 20 days of the initial dose or received the second mRNA-1273 dose within 27 days of the initial dose. In addition, individuals who were booster vaccinated within five months of their second vaccination and individuals without valid vaccination cards were excluded from the analysis. Individuals with a history of conditions such as HIV, AIDS, lupus, cancer or rheumatoid arthritis, or transplantation of bone marrow or solid organs who were on medications such as immunosuppressants, steroids, or chemotherapeutic agents were also excluded. Finally, university students and employees vaccinated before March 31, 2021, and before March 8, 2021, respectively, were excluded from the analysis.
The sample size for analysis was based on the propensity scores of the participants based on their vaccination status: unvaccinated or vaccinated with two doses or three (booster) doses of the mRNA-1273 or BNT162b2 vaccines within the previous five months. The other variables considered for propensity score matching included sex, age, student affiliation (residential or non-residential housing), employee type (staff or faculty), race, self-reported medical history (hypertension, cardiovascular illness, diabetes, obesity, renal disease, neurological disorders, hepatic disease, or pulmonary disease), tobacco or nicotine product usage, number of COVID-19 tests during university semesters of Fall 2020, Spring 2021, and Fall 2021, and COVID-19 history.
Results
Based on the propensity scores, 1,944 and 658 university students and university employees, respectively, were selected for the analysis. Among the students, 49% were unvaccinated, 25.5% were fully vaccinated, and 25.5% had received booster vaccination. About 28% of the students tested SARS-CoV-2-positive during follow-up and significantly lower positivity rates were observed among booster-vaccinated students (16%) in comparison to unvaccinated students (31%) and doubly vaccinated students (35%).
Among the employees, 52% were unvaccinated, 13% were fully vaccinated, and 35% were booster vaccinated. About 21% of the employees tested SARS-CoV-2-positive during follow-up, and the positivity rates were significantly lower among booster-vaccinated employees (9.6%) in comparison to unvaccinated employees (28%) and fully vaccinated employees.
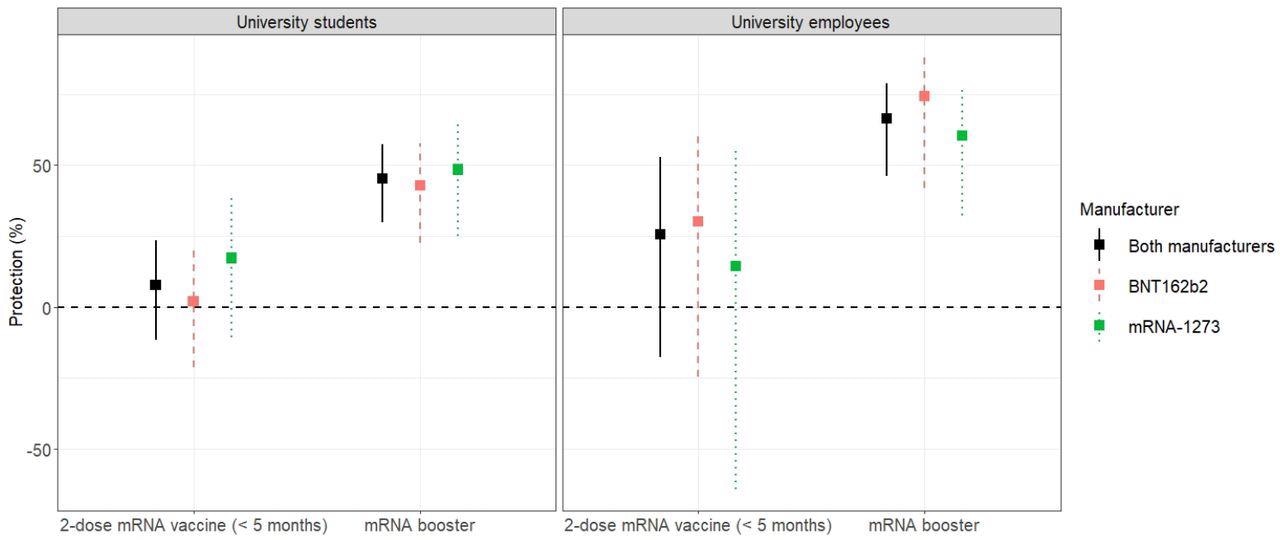
Estimates of vaccine protection against any SARS-CoV-2 infection caused by the omicron variant in university students (left) and employees (right).
Booster vaccination conferred 66.4% and 45.4% protection against SARS-CoV-2 infections among the university employees and among the university students, respectively.
In comparison to the two-dose mRNA vaccination, the estimated increase in immune protection by the vaccine boosters was 41% and 37.7% among the employees (P=0.024) and among the students (P=0.001), respectively.
However, there was no statistically significant conclusive evidence on the protection conferred by the two doses of the mRNA vaccines, the waning of serological immunity within five months of double or triple mRNA vaccination, and the variability in immune protection conferred by previous SARS-CoV-2 infections. Further, there was no statistically significant difference in the immune protection conferred by heterologous or homologous mRNA-1273 or BNT16b2 vaccinations among university students or employees.
According to the results of the study, mRNA boosters confer moderate immunity against Omicron infections, as well as substantially enhancing the immunity conferred by two doses of the mRNA vaccines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
COVID-19 vaccine effectiveness against general SARS-CoV-2 infection from the omicron variant: A retrospective cohort study. Lior Rennert, PhD, Zichen Ma, PhD, Christopher S. McMahan, PhD, Delphine Dean, PhD, medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.06.22274771, https://www.medrxiv.org/content/10.1101/2022.05.06.22274771v1
- Peer reviewed and published scientific report.
Rennert, Lior, Zichen Ma, Christopher S. McMahan, and Delphine Dean. 2023. “Covid-19 Vaccine Effectiveness against General SARS-CoV-2 Infection from the Omicron Variant: A Retrospective Cohort Study.” Edited by Patrick DMC Katoto. PLOS Global Public Health 3 (1): e0001111. https://doi.org/10.1371/journal.pgph.0001111. https://journals.plos.org/globalpublichealth/article?id=10.1371/journal.pgph.0001111.