Prior studies indicated that CoV disease 2019 (COVID-19) and diabetes mellitus (DM) could lead to chronic and acute inflammation. Moreover, both disease states can influence the outcome and clinical course of the other. COVID-19 caused acute kidney injury (AKI) in more than 20% of hospitalized SARS-CoV-2-infected individuals, and those with pre-existing diabetes had higher fatality rates.
The authors of the present research previously showed that SARS-CoV-2 infects both vascular and kidney organoids produced from human pluripotent stem cells (hPSCs), and the addition of clinical-grade human recombinant soluble angiotensin-converting enzyme 2 (ACE2) inhibits this interaction. Nonetheless, organoids that could model human co-morbidities linked with severe COVID-19, like diabetes, do not exist despite their importance in SARS-CoV-2 studies.
Study: A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells. Image Credit: Cell Press
About the study
The present research aimed to determine why diabetes patients were more likely to acquire severe SARS-CoV-2 infection by establishing a human kidney organoid model demonstrating early signs of diabetic renal disease development.
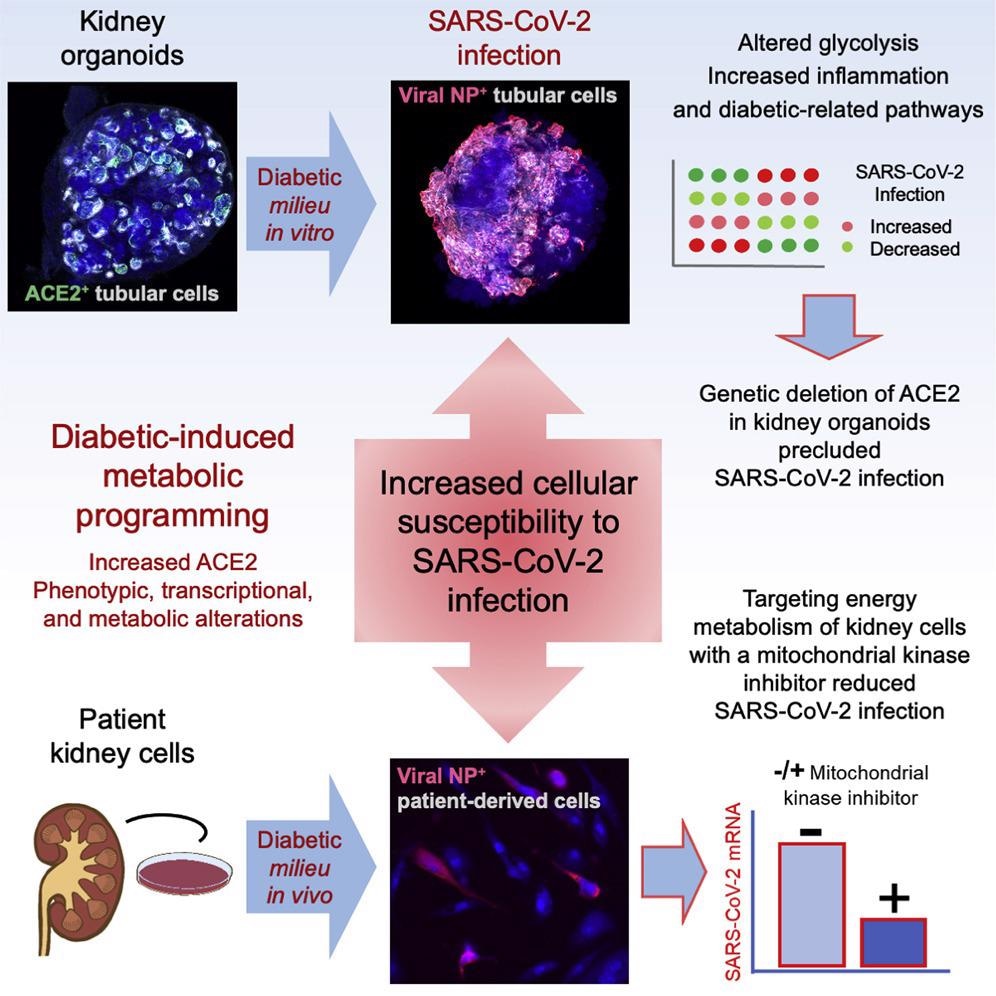
The scientists adapted their earlier approach for establishing hPSCs-derived diabetes-like kidney organoids to mimic in vitro fluctuations in glucose concentrations similar to the diabetic patients. They devised a culture system using persistent low and high glucose levels in an oscillatory manner. Diabetic and control kidney organoids were challenged with SARS-CoV-2, retrieved, and evaluated one day after infection.
Additionally, the authors created ACE2 knockout (KO) hPSC lines using clustered regularly interspaced short palindromic repeats (CRISPR)- associated protein 9 (Cas9) genome editing. Further, the essentiality of ACE2 in SARS-CoV-2 infections in kidney organoids was determined using ACE2 KO hPSC lines.
To further confirm the specific function of ACE2 in SARS-CoV-2 infection in kidney organoids, the team generated basigin (BSG) and non-expressor of pathogenesis-related genes 1 (NPR1) KO hPSCs using CRISPR-Cas9. They assessed whether ACE2 was required for the reported rise in the kidney SARS-CoV-2 infections under diabetic culture conditions. The investigators used kidney human proximal tubular cells (HPTCs) obtained from renal biopsies of diabetic and non-diabetic individuals to estimate if the COVID-19-linked kidney damage was caused by indirect kidney injury responses or direct viral infection of target kidney cells.
Results
The study results showed ACE2 positivity in renal tubular-like cells utilizing confocal microscopy while evaluating kidney organoids for the expression of the SARS-CoV-2 entrance receptor ACE2, backing prior investigations. The current findings confirmed earlier reports that a high oscillatory glucose environment promotes ACE2 expression at both the protein and messenger ribonucleic acid (mRNA) levels.
In the diabetic kidney organoids, an elevated SARS-CoV-2 titer was found at the protein and mRNA levels, parallel with increased ACE2 expression. A significant reduction in ACE2 mRNA expression occurred upon SARS-CoV-2 infection, consistent with earlier findings employing ileum- and colon-derived human intestinal organoids. In diabetic organoids, single-cell profiling revealed a reduction in oxidative phosphorylation (OXPHOS) and glycolytic-based metabolism and depicted a hypoxic fingerprint in response to COVID-19.
Although retention of renal differentiation occurred in ACE2 KO hPSC-derived kidney organoids during SARS-CoV-2 infection, these ACE2 defective organoids demonstrated changes in lipid metabolism, OXPHOS, endothelium, and angiogenesis, congruent with the previously documented roles of ACE2 in COVID-19. Furthermore, transmission electron microscopy (TEM) analysis and confocal microscopy revealed that BSG and NPR1 KO kidney organoids tolerated viral infection, ruling out the significance of NPR1 and BSG in kidney SARS-CoV-2 infections.
The researchers found that aberrations in ACE2 expression and cellular metabolism in kidney organoids associated with elevated glucose concentrations were directly linked to increased SARS-CoV-2 loads after infection. These abnormalities might cause a transition from an OXPHOS to an aerobic glycolytic mode, thus increasing vulnerability to SARS-CoV-2 infection. Similarly, diabetic kidney proximal tubular cells had elevated modified mitochondrial respiration and glycolysis, corresponding with high SARS-CoV-2 infections relative to non-diabetic samples. Kidney SARS-CoV-2 infections were reduced following exposure to dichloroacetate (DCA), a metabolic modulator that promotes mitochondrial OXPHOS by inhibiting glycolysis.
Conclusions
To summarize the study findings, high glucose oscillations in constructed human kidney organoids caused transcriptional, metabolic, and phenotypic changes similar to those seen in diabetic kidney disease. This diabetic milieu augmented SARS-CoV-2 infection and ACE2 expression.
Diabetic-like kidney organoids had greater SARS-CoV-2 loads after viral infection than the control kidney organoids. In kidney organoids under diabetic-like or control settings, genetic deletion of ACE2, yet not BSG or NRP1, inhibited virus detection. In addition, cells obtained from diabetic individuals' kidney biopsies had increased glycolysis and changed mitochondrial respiration, leading to superior SARS-CoV-2 infections than non-diabetic cells. On the contrary, exposure to an inhibitor of aerobic glycolysis named DCA lowered SARS-CoV-2 infections in patient cells.
Altogether, the current research offered mechanistic evidence for metabolic changes that promote cellular sensitivity to SARS-CoV-2 infections and firmly identified ACE2 as the essential human kidney SARS-CoV-2 receptor, even in diabetic settings. The present data deepen the understanding of the diabetes-mediated metabolic changes in the kidney in enhancing SARS-CoV-2 infection vulnerability, paving the way for establishing novel therapies in COVID-19 pathogenesis addressing energy metabolism.