Immunocompromised individuals are highly vulnerable to severe coronavirus disease 2019 (COVID-19) and, therefore, are the highest priority for COVID-19 vaccination. However, detailed knowledge of the reactogenicity of COVID-19 mRNA vaccines among immunocompromised individuals is limited.
 Study: SARS-CoV-2-mRNA Booster Vaccination Reverses Non-Responsiveness and Early Antibody Waning in Immunocompromised Patients – A Phase Four Study Comparing Immune Responses in Patients With Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease. Image Credit: KT Stock photos / Shutterstock
Study: SARS-CoV-2-mRNA Booster Vaccination Reverses Non-Responsiveness and Early Antibody Waning in Immunocompromised Patients – A Phase Four Study Comparing Immune Responses in Patients With Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease. Image Credit: KT Stock photos / Shutterstock
About the study
In the present phase IV study, researchers evaluated the humoral/antibody/B lymphocyte-mediated and cell (T lymphocyte)-mediated immune responses induced by primary and booster vaccinations of the mRNA-1273 and the BNT162b2 vaccines among immunocompromised MM, IBD, or SOT patients.
The patients were enrolled between March 2021 and June 2021 and did not have a history of prior SARS-CoV-2 infections or vaccination. They were doubly vaccinated with BNT162b2 or mRNA-1273, with the two doses administered three and four weeks apart, respectively. Blood samples were obtained from all participants before the first vaccination, on the second vaccination day, four weeks post the second vaccination, and five to six months post the second vaccination. In addition, blood was drawn from the patients four weeks post the booster vaccination.
Peripheral blood mononuclear cells (PBMCs) obtained before and after a week of primary vaccination were analyzed for T lymphocyte responses. In addition, the expression of cytokines such as interferon-gamma (IFN-ꓬ), interleukins (IL)-2,5,10,17a,22, and granulocyte-macrophage colony-stimulating factor (GM-CSF) was evaluated.
Further, the titers of SARS-CoV-2 spike protein subunit 1 (S1)-specific immunoglobulin G (IgG) antibodies were evaluated using enzyme-linked immunosorbent assays (ELISA). The titers were expressed as binding antibody units (BAU/ml), and the mean antibody titer values were expressed as geometric mean titers (GMT) or geometric mean concentrations (GMC). In addition, neutralization assays and flow cytometry assessments were performed.
Results
A total of 263 immunocompromised patients with SOT (n=63), MM (n=70), or IBD (n=130) were analyzed and compared to 66 controls. Post primary vaccination, the GMTs were substantially lower among SOT (GMT=100 BAU/ml) and MM patients (GMT=72 BAU/ml) compared to controls (GMT=453 BAU/ml).
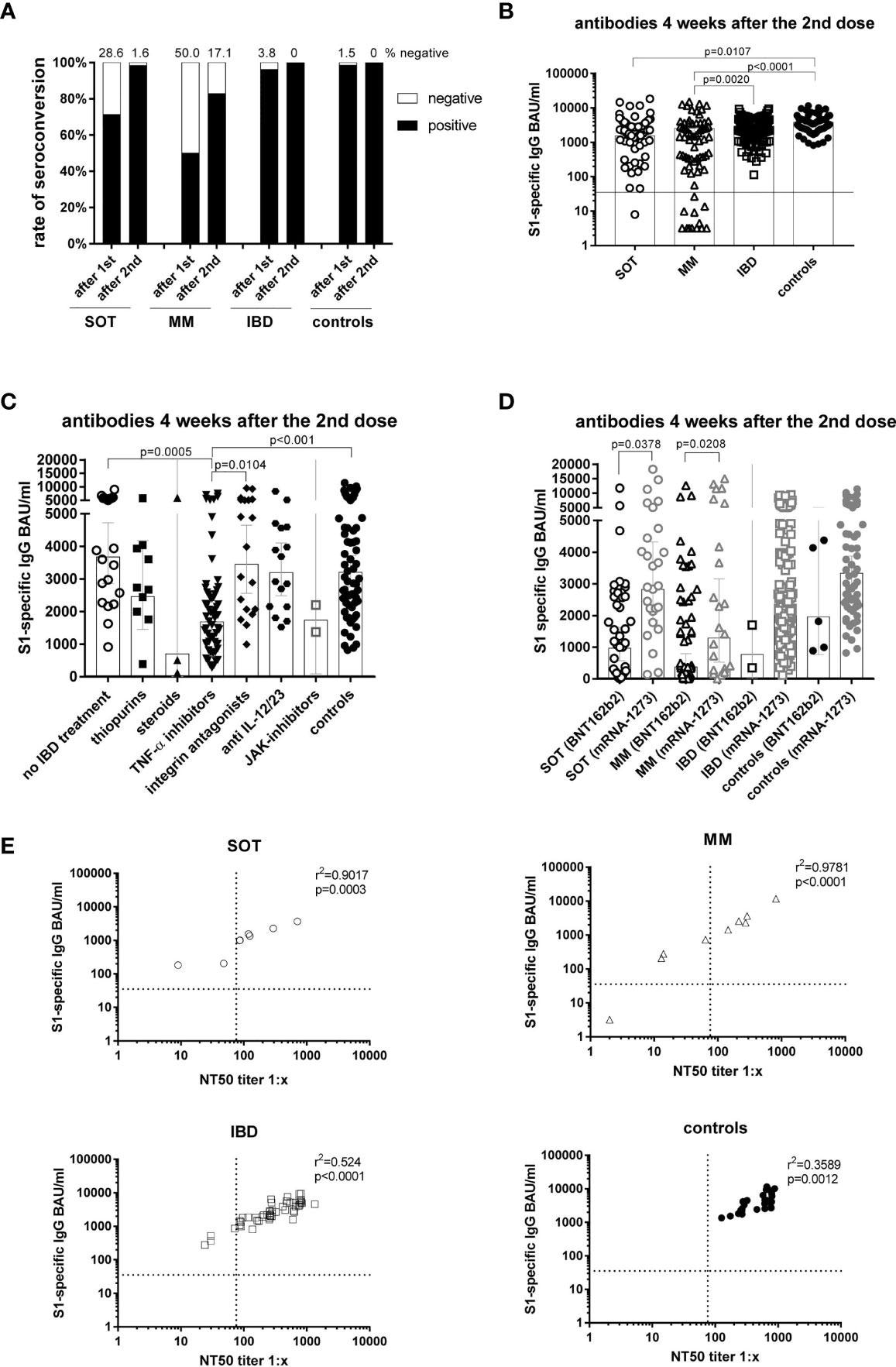
Antibody responses four weeks after the second mRNA vaccination and correlation of S1-specific IgG with neutralizing antibodies. Seroconversion rates after the first and second dose in all study participants of all groups (A). Individual S1-specific IgG levels of all participants (B). S1-specific IgG levels of IBD patients in respect of their treatment and in comparison to the controls (C). S1-specific antibody levels in relation to the type of mRNA vaccine applied (BNT162b2 or mRNA-1273), whereby due to the number of participants statistical differences could only be calculated for SOT and MM patients (D). Correlation of S1-specific IgG levels with the NT50 of a neutralization test with sera taken four weeks after the second vaccination (n=106) (E). SOT (n=63) are represented as circles, MM (n=70) as triangles, IBD (n=130) patients as squares and controls (n=55) as full black or grey circles. Differences between the groups below p values of 0.05 were regarded as significant. The black and dotted lines (E) indicate the threshold for positive results (35.2 BAU/ml and NT50). Bars (B-D) represent GMC with 95% confidence interval (CI).
Four weeks post second vaccination, the lowest GMCs were observed among MM patients (GMC=553 BAU/ml) in comparison to IBD (GMC=2275 BAU/ml), SOT patients (GMC=1529 BAU/ml), and controls (GMC=3206 BAU/ml). Among the IBD patients, those treated with TNF-α inhibitors demonstrated significantly lower antibody titers (GMC=1685 BAU/ml) than untreated IBD patients (GMC=3676 BAU/ml), vedolizumab-treated IBD patients (GMC=3454 BAU/ml) and controls (GMC=3206 BAU/ml).
Five to six months post the second vaccination, the highest percentage of seronegative individuals was observed among the MM patients (18%) followed by SOT (10%) and IBD (4%) patients, whereas all controls remained seropositive. High seronegativity was associated with low counts of a cluster of differentiation 19 positive (CD19+) B lymphocytes.
Further, antibody waning (mean S1-specific IgG fold-decreases) was highest among IBD patients (12-fold) and MM patients (11.7-fold) followed by SOT patients (seven-fold) in comparison to controls (five-fold). IBD and cancer patients demonstrated lower antibody titers than controls, with the lowest antibody titers detected among IBD patients treated with TNF-α inhibitors (19-fold decrease) compared (6.3-fold). S1-specific IgG titers were found to correlate with the IFN-ꓬ and IL-2 cytokine expression among IBD patients and controls but not among cancer patients.
Among SOT and MM patients, antibody titers were higher among those vaccinated with mRNA-1273 (MM: GMC=1289 BAU/ml, SOT: GMC=2827 BAU/ml,) compared to those vaccinated with BNT162b2 (MM: GMC=375 BAU/ml, SOT: GMC=965 BAU/ml). All (except one) non-responders were vaccinated with BNT162b2. Furthermore, cancer patients maintained higher GMCs with the mRNA-1273 vaccine compared to the BNT162b2 vaccine (SOT: 510 vs. 145; MM: 215 vs. 140). This indicated that the mRNA-1273 vaccine was more immunogenic than the BNT162b2 vaccine.
Of note, booster vaccination elevated antibody titers by more than eight-fold among seroresponders of all groups and induced anamnestic immune responses even among those with undetectable antibody titers prior to booster vaccinations. This was indicative of the enhanced immune protection conferred by the vaccine boosters. Nevertheless, even post booster vaccination, the antibody titers among IBD patients treated with TNF-α inhibitors continued to be lower compared to untreated IBD patients and controls.
On phenotyping leukocytes in the flow cytometry analysis, MM patients demonstrated lower total absolute leukocyte counts, total lymphocyte counts, CD3+ T cells, and CD3+ CD4+ T helper cells in comparison to controls. MM and SOT patients demonstrated lower levels of CD19+ B lymphocytes than controls. However, no significant differences were observed in the counts of granulocytes, monocytes, NK cells, and CD8+ T cells, except for lower granulocyte counts in MM patients in comparison to controls. Lower lymphocyte counts were detected in cancer patients but not in IBD patients.
Conclusion
Overall, the study findings showed that the booster vaccination enhanced immune protection among the immunocompromised individuals against SARS-CoV-2. In addition, the mRNA-1273 vaccine demonstrated higher immunogenicity than the BNT162b2 vaccine among MM, SOT, and IBD patients.
Journal reference:
- Wagner A, Garner-Spitzer E, Schötta A-M, Orola M, Wessely A, Zwazl I, Ohradanova-Repic A, Weseslindtner L, Tajti G, Gebetsberger L, Kratzer B, Tomosel E, Kutschera M, Tobudic S, Pickl WF, Kundi M, Stockinger H, Novacek G, Reinisch W, Zielinski C and Wiedermann U (2022) SARS-CoV-2-mRNA Booster Vaccination Reverses NonResponsiveness and Early Antibody Waning in Immunocompromised Patients – A Phase Four Study Comparing Immune Responses in Patients With Solid Cancers, Multiple Myeloma and Inflammatory Bowel Disease. Front. Immunol. 13:889138. doi: 10.3389/fimmu.2022.889138, DOI: https://doi.org/10.3389/fimmu.2022.889138, https://www.frontiersin.org/articles/10.3389/fimmu.2022.889138/full?utm_source=S-TWT