Highly infectious and transmissible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs) are still emerging. Hence, there is an urgent need to increase population-based vaccine rates and suppress breakthrough infections.
Children were initially not vaccinated for SARS-CoV-2, but now they constitute the most adversely affected age group. It is worrisome that they are at a higher risk of developing multi-system inflammatory syndrome (MIS-C) after exposure to SARS-CoV-2, pointing to the importance of vaccination for all age groups. Subsequently, there is an urgent need to vaccinate them to prevent fatal health outcomes and stop community-level SARS-CoV-2 spread.
All the available messenger ribonucleic acid (mRNA)-based COVID-19 vaccines have been given emergency use authorization (EUA) for children five years and older. However, in the absence of clinical data supporting their safe use in children, children under 12 years are administered an adjusted lower vaccine dose.
It is unknown how the vaccine dose adjustment impacts immunogenicity and the outcome in children. A child's immune system is inexperienced and elicits a humoral response that matures in response to vaccines, including mRNA vaccines over the years. However, this response can differ from the immune response in adults.
About the study
In the present study, researchers comprehensively profiled humoral immune response in 32 children between five and 11 years who received two doses of 10μg mRNA-based BNT162b2 vaccine. The median age of these children was nine years, and 34% of them were female.
They compared the vaccine-induced functional humoral immune response of the children to 30 adolescents in the age group of 12-15 years and nine adults over 16 years, both receiving the adult-recommended 30 μg vaccine dose.
The team collected plasma samples before the first (V0) and second vaccine doses (V1). Later, they also collected samples between two to four weeks after the second (V2) vaccine dose. They measured and analyzed titers of immunoglobulins (Ig) M, A, and G1 specific to the SARS-CoV-2 spike (S) and receptor-binding domain (RBD).
Study findings
The magnitude of humoral immune responses across the three study groups was markedly different. In children, vaccination-induced slower antibody production and lower antibody titers; however, the antibodies were functionally robust and conferred protection against severe disease across all SARS-CoV-2 VOCs. Conversely, the fragment, crystallizable (Fc) effector functions to Omicron were lowest in children who received the 10 μg vaccine dose.
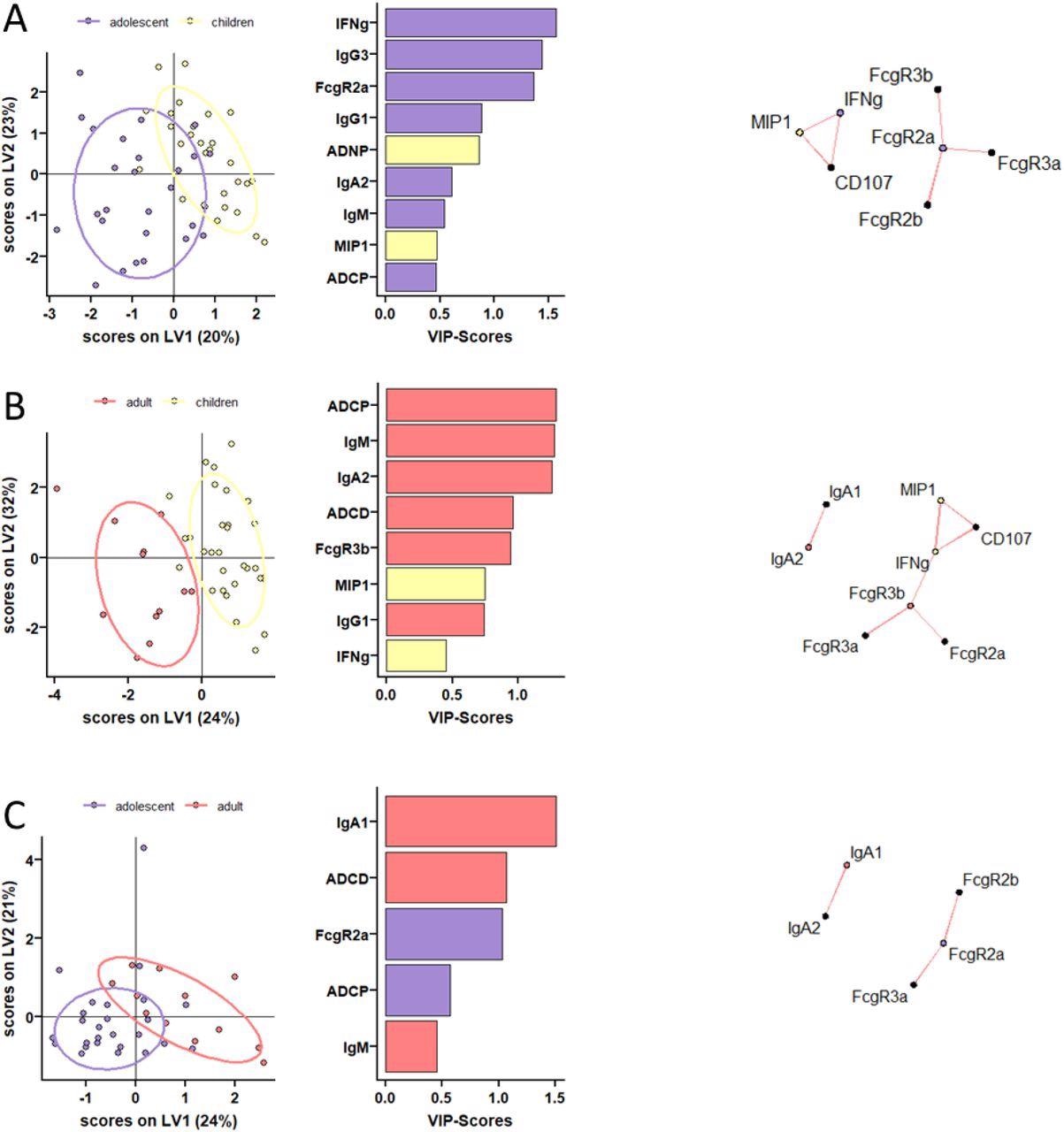
Distinct humoral profiles between BNT162b2 mRNA vaccinated pediatric children, adolescent children, and adults against SARS-CoV-2 wild-type spike. (A-C) A machine learning model was build using LASSO selected SARS-CoV-2 wild-type spike specific features at V2. Vaccine response in adolescent children (purple) and pediatric children (yellow) given 10 μg BNT162b2 (A), in adults (red) and pediatric children (yellow) (B), or in adults (red) and adolescent children (purple) given 30 μg BNT162b2 (C) were compared. Separation of the groups in the PLS-DA is shown in the right panel. Variable importance (VIP) score of LASSO selected features is shown in the bar graph and features are colored coded by the group they were enriched in. The network plots (right panel) show significant (p<0.05) Spearman correlations (|r|>0.7, only positive correlations were observed) to other (non-selected) features.
At V0, children had lower but detectable S and RBD-specific IgA and IgG titers. At V1, although a majority of children under 11 years immunized with 10 μg BNT162b2 seroconverted, their S and RBD-specific IgG1 titers remained significantly lower compared to two other groups. Even after V2, IgG1 titers remained low in young children. Analysis of the binding properties of Fc receptor (FcR) of SARS-CoV-2 S-specific antibodies across the three study groups revealed interesting insights.
All children in the age group of five to 11 years mounted similar levels of FcR binding to adults across FcγRs, albeit the responses were quite variable. Overall, children generated superior functional antibodies compared to adults. In particular, after the second dose, the generated antibodies were superior at inducing antibody-dependent neutrophil phagocytic antibodies (ADNP). Additionally, vaccine-induced similar levels of antibody-dependent cellular monocyte phagocytosis (ADCP) compared to adolescents and adults.
The authors observed detectable levels of antibodies mediating additional Fc effector functions to Omicron in adults over 16 years. Conversely, children receiving lower vaccine doses elicited antibodies with comparable cross-reactivity but reduced functionality against Omicron.
Furthermore, BNT162b2 vaccination augmented pre-existing immunity and generated novel human coronavirus OC43 specific antibodies across the three study groups. However, future studies should evaluate whether vaccination restricted the antibodies generated in response to related SARS-CoV-2 VOCs, e.g., after natural infection.
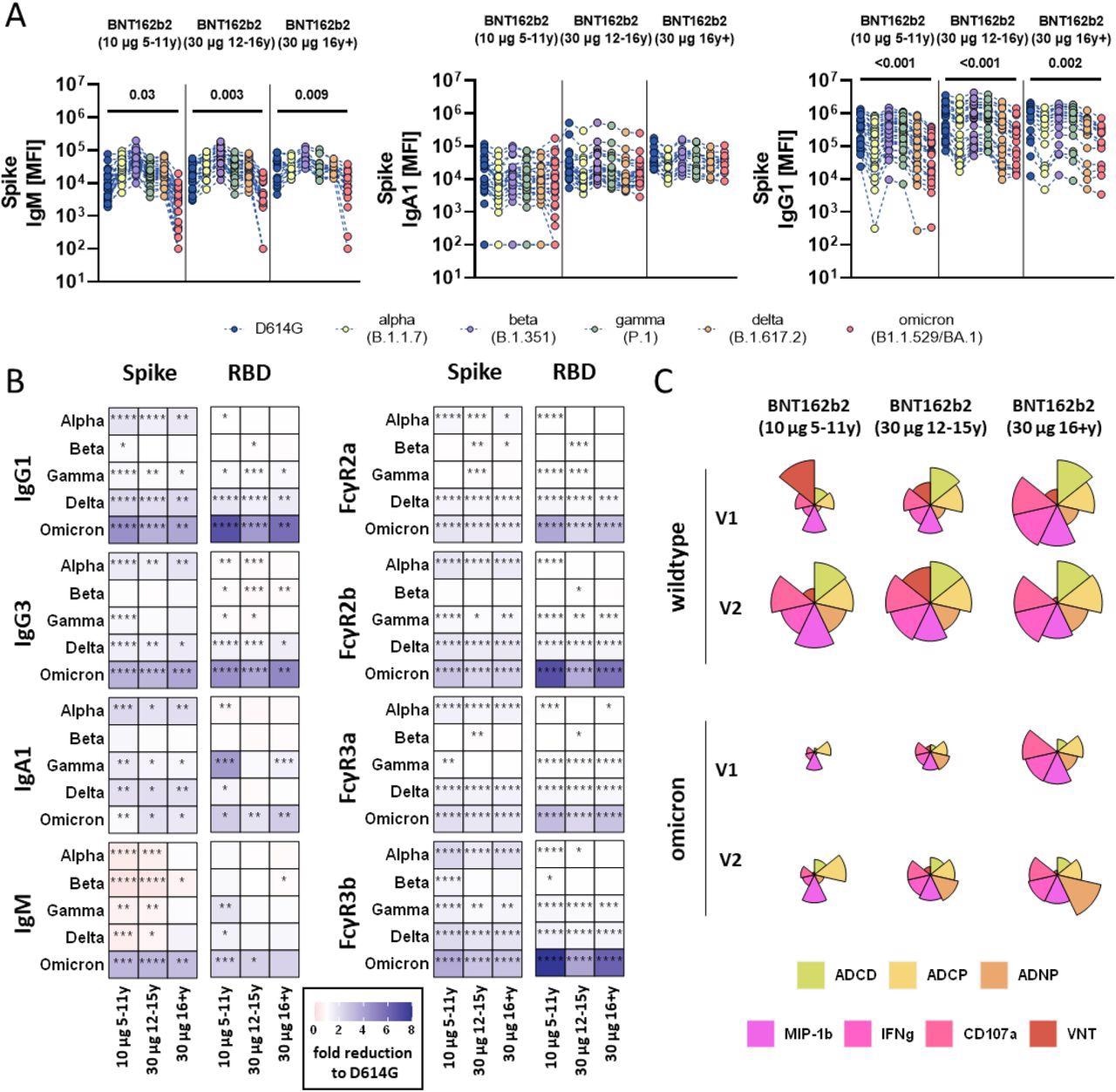
SARS-CoV-2 variants of concern specific humoral immune responses after vaccination with BNT162b2. (A) Vaccine-induced IgM, IgA1, and IgG1 response to D614G (wild-type; blue), alpha (B.1.1.7, yellow), beta (B.1.1.7, purple), gamma (P.1, green), delta (B.1.617.2, orange), and omicron (B1.1.529/BA.1, red) to the full Spike in children receiving 10μg of BNT162b2 (ages 5-11 years old, n =) or adolescent receiving 30 μg BNT162b2 (ages 12-15 years old, n=) or adults (16+ years old, n=) at V2. A two-sided Kruskal-Wallis test with Benjamini-Hochberg correction for multiple testing was performed to compare D614G and omicron-specific antibody titers. P-values for significant different comparisons are shown above the dataset. B) Heatmaps show the fold change (red=increase, blue= decrease) for the different VOCs compared to the original D614G variant for Spike and RBD specific IgG1, Ig3, IgA1 and IgM titers or binding to FcγR2a, FcγR2b, FcγR3a, FcγR3b. C) Flower plots summarize ADCD, ADCP, ADNP, ADNKA (CD107a, IFNγ, MIP-1β) and neutralization (VNT) at V1 and V2 against D614G (upper panel) or omicron (lower panel) Spike in 10μg of BNT162b2 (ages 5-11 years old) or adolescent receiving 30 μg BNT162b2 (ages 12-15 years old) or adults (16+ years old) at V2. Each petal represents a specific function (compare color key) and the length of the petal corresponds of the intensity of Z-scored and normalized data. Asterisks in B) indicate significant differences of the respective variant in a paired two-sided Wilcoxon rank test. P-values were corrected for multiple testing using Benjamini-Hochberg correction. *:p<0.05, **:p<0.01,***:p<0.001, ****:p<0.0001.
Conclusions
Overall, a three-fold lower dose of BNT162b2 vaccination resulted in a variable response in five to 11 years old children. Its two-dose regimen specifically elevated levels of opsonophagocytic and natural killer (NK) cell-activating antibodies implicated in the resolution of severe disease. Furthermore, antibody avidity was higher in young children.
Interestingly, a variation in epitope selection or post-translational modification of the antibody Fc-domain most likely altered the quality of the pediatric antibody quality. Future studies should critically monitor the stability of the more variable response in young children to inform immunization guidelines and strategies in children.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
BNT162b2 induces robust cross-variant SARS-CoV-2 immunity in children, Yannic C Bartsch, Jessica W Chen, Jaewon Kang, Madeline D Burns, Kerri J St.Denis, Maegan L Sheehan, Jameson P Davis, Alejandro B Balazs, Lael M Yonker, Galit Alter, medRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.05.18.22275283, https://www.medrxiv.org/content/10.1101/2022.05.18.22275283v1
- Peer reviewed and published scientific report.
Bartsch, Yannic C., Jessica W. Chen, Jaewon Kang, Madeleine D. Burns, Kerri J. St Denis, Maegan L. Sheehan, Jameson P. Davis, et al. 2022. “BNT162b2 Induces Robust Cross-Variant SARS-CoV-2 Immunity in Children.” Npj Vaccines 7 (1): 1–10. https://doi.org/10.1038/s41541-022-00575-w. https://www.nature.com/articles/s41541-022-00575-w.