Only a few studies have evaluated oral antivirals against the Coronavirus Disease 2019 (COVID-19) virus in patients without supplemental oxygen. In the absence of supporting data, the medical community has prioritized the distribution of these drugs to those at the highest risk of disease progression only, e.g., not fully vaccinated elderly with multiple pre-existing comorbidities.
 Study: Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study. Image Credit: NIAID
Study: Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present observational study, researchers enrolled hospitalized COVID-19 patients in Hong Kong between 26 February 2022 and 5 May 2022. The study patients were not provided supplemental oxygen but received either molnupiravir or nirmatrelvir/ritonavir treatment.
They were either admitted to hospitals within three days of their COVID-19 diagnosis or had confirmed COVID-19 within three days of their admission. This helped the researchers account for any potential time lag in confirming COVID-19.
The date of hospital admission (day 0) was the index date. Likewise, the researchers defined the treatment exposure period as two days after hospital admission. The team followed up with the patients from the index date until death or other outcome events, a crossover of oral antiviral treatment, or the end of the study period.
The team selected study controls not receiving oral antivirals but hospitalized for COVID-19, with a propensity score ratio of 1:4. They compared the test and control group patients for changes in their clinical status, including in-hospital death, initiation of supplemental oxygen, not requiring oxygen support, and hospital discharge.
The researchers also performed logistic regression on study baseline covariates, such as gender, age, symptom onset, and Charlson comorbidity index (CCI), to estimate the propensity of receiving each drug. Using the standardized mean difference (SMD), the team assessed the balance of each baseline covariate between the test and control groups before and after propensity-score matching. The SMD greater than 0.1 indicated covariate imbalance.
Further, the team used the Cox regression model to estimate hazard ratios (HRs) of event outcomes with 95% confidence intervals (CI) between oral antiviral users and controls. After re-matching baseline covariates of the two study groups, they further analyzed the effectiveness of molnupiravir and nirmatrelvir/ritonavir on each outcome.
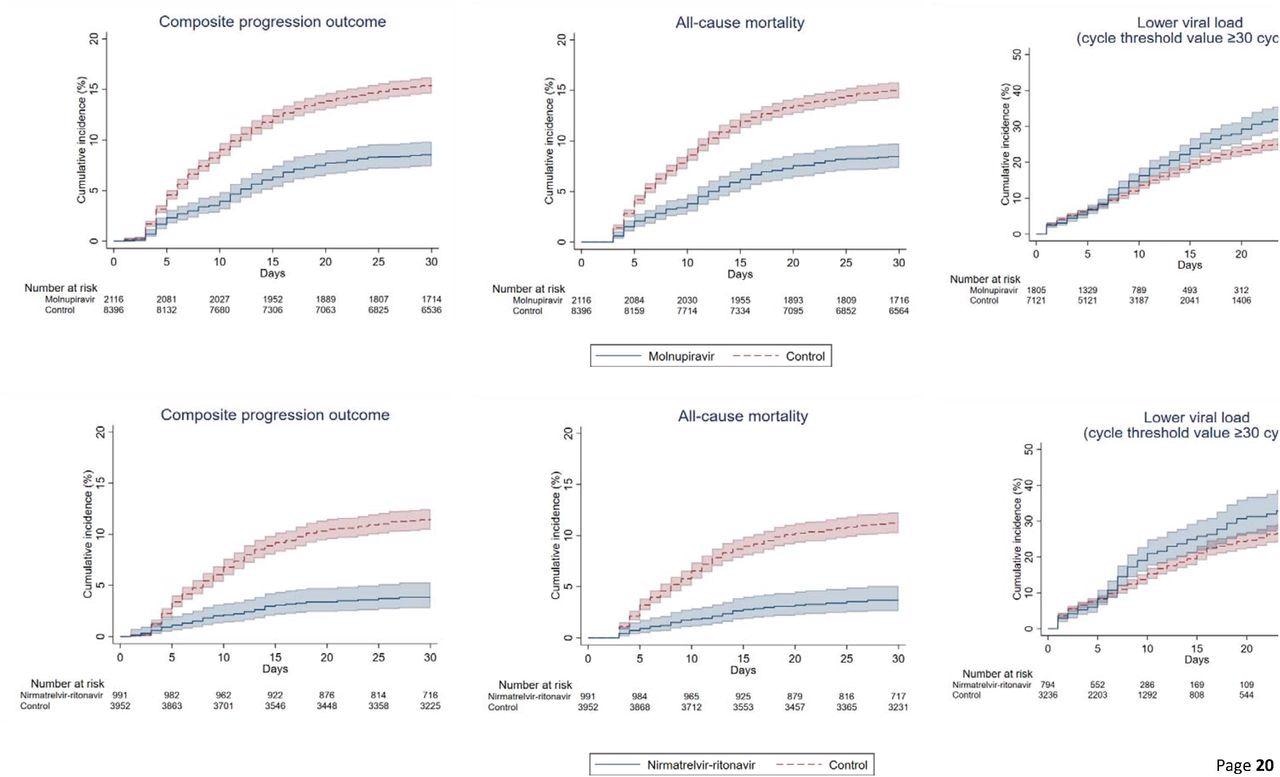
Cumulative incidence plots of (a) composite progression outcome, (b) all-cause mortality, and (c) lower viral load for molnupiravir users versus t matched controls, and (a) composite progression outcome, (b) all-cause mortality, and (c) lower viral load for nirmatrelvir/ritonavir users versus their match controls
Study findings
Over an average follow-up of 41.3 days, there were 40,776 hospitalized patients with confirmed COVID-19 between February and April 2022. Of these, 2,359 and 1,000 patients were prescribed molnupiravir and nirmatrelvir/ritonavir during hospital admission, respectively. After 1:4 propensity-score matching, there were 2,116 molnupiravir users with 8,396 matched control and 991 nirmatrelvir/ritonavir users with 3,952 matched control.
The proportion of patients who received 800mg molnupiravir twice daily for five days was 96.2%, while the proportion of patients who completed the five-days regimen of 300 mg nirmatrelvir with 100mg of ritonavir twice daily was 98.5%.
All-cause mortality (crude) incidence rates among those on molnupiravir were 22.24 and 1.06 events per 10,000 person-days. Likewise, for nirmatrelvir/ritonavir users, it was 11.04 and 1.75 events per 10,000 person-days. Additionally, molnupiravir users had a reduced risk of invasive mechanical ventilation (IMV), with an HR of 0.31, 95% CI. The time to attain lower viral load was substantially shorter among patients prescribed antivirals than matched controls. The HRs for the same among molnupiravir users was 1.21, and nirmatrelvir/ritonavir users were 1.25.
The in-hospital death was noticeably higher in the control group relative to antiviral users on day-3 from baseline. Accordingly, the proportion of individuals with in-hospital deaths was 1.4% vs. 0.6% for molnupiravir users and 1.1% vs. 0.4% for nirmatrelvir/ritonavir users, compared to controls. A similar pattern persisted till day-28 of the follow-up; the proportion of individuals with in-hospital deaths was 14.8% vs. 8.3% for molnupiravir users and 10.3% vs. 3.2% for nirmatrelvir/ritonavir users. After 1:1 propensity matching, the authors noted a greater risk of mortality (HR=1.53) and longer length of hospital stay (0.83 days) among molnupiravir users.
Conclusions
Oral antiviral drugs, molnupiravir and nirmatrelvir/ritonavir lowered the risk of disease progression and all-cause mortality even against Omicron sub-variant BA.2 in real-world settings. Additionally, they dropped viral load quicker than matched controls. Molnupiravir treatment also lowered the risk of initiating IMV; likewise, nirmatrelvir/ritonavir therapy shortened the length of hospital stay.
A direct comparison showed that nirmatrelvir/ritonavir treatment reduced the mortality risk more than molnupiravir use. Overall, the study demonstrated that these oral antivirals could treat people at higher risk of severe COVID-19. However, further research is needed to inform the safety and effectiveness of oral antivirals in specific settings, populations, and healthcare settings.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong's Omicron BA.2 wave: an observational study, Carlos KH Wong, Ivan CH Au, Kristy TK Lau, Eric Lau, Benjamin J Cowling, Gabriel M Leung, medRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.05.19.22275291, https://www.medrxiv.org/content/10.1101/2022.05.19.22275291v1
- Peer reviewed and published scientific report.
Wong, Carlos K H, Ivan C H Au, Kristy T K Lau, Eric H Y Lau, Benjamin J Cowling, and Gabriel M Leung. 2022. “Real-World Effectiveness of Early Molnupiravir or Nirmatrelvir–Ritonavir in Hospitalised Patients with COVID-19 without Supplemental Oxygen Requirement on Admission during Hong Kong’s Omicron BA.2 Wave: A Retrospective Cohort Study.” The Lancet Infectious Diseases, August. https://doi.org/10.1016/s1473-3099(22)00507-2. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00507-2/fulltext.