The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is capable of infecting people of any age. Although COVID-19 is often mild in young children relative to adults, thousands of children have been admitted to hospitals in the United States (US) owing to SARS-CoV-2 infection, with around one-third of them having no prior medical issues. Over 800 US children aged 0 to 11 years have died from COVID-19, and during the 2021/2022 fall/winter SARS-CoV-2 outbreak in the US, children constituted more than 25% of COVID-19 cases. Moreover, COVID-19 rarely produces a multisystem inflammatory disease in children (MIS-C).
While SARS-CoV-2 messenger ribonucleic acid (mRNA) vaccinations are available for children aged five or older, no vaccine for children under five years has been approved or recommended. Further, the drawback of the current SARS-CoV-2 mRNA and other parenteral vaccines is that they did not directly induce immunity in the respiratory tract, where SARS-CoV-2 replication, entrance, egress, and disease occur primarily. Therefore, pediatric COVID-19 vaccines that impart systemic and mucosal immunity are required.
About the study
In the present work, the scientists developed B/HPIV3/S-6P, a live-attenuated chimeric bovine/human parainfluenza virus type-3 (B/HPIV3)-vectored COVID-19 vaccine option harboring prefusion-stabilized SARS-CoV-2 spike (S) protein. Interestingly, the B/HPIV3 vector was developed initially as a pediatric vaccination prospect for a negative-sense single-stranded RNA virus, HPIV3, a leading cause of respiratory disease, notably in babies and young children under five years.
The authors of the present research recently demonstrated that B/HPIV3 containing perfusion-stabilized SARS-CoV-2 S protein effectively safeguarded hamsters from a vaccine antigen-matched SARS-CoV-2 isolate infection, deterring lung inflammation, weight loss, and proficiently lowering SARS-CoV-2 replication in the lower and upper respiratory tract.
In this study, the team tested the immunogenicity, safety, and protective capacity of a single dose of intratracheal/intranasal (IT/IN) B/HPIV3/S-6P vaccination in rhesus macaques (RMs). They determined SARS-CoV-2 S-specific T-cell, systemic, and mucosal antibody responses for assessing immunogenicity. In addition, they analyzed neutralizing antibody responses of B/HPIV3/S-6P to the vaccine-matched SARS-CoV-2 strand and the SARS-CoV-2 Omicron BA.1, Delta, Beta, and Alpha variants of concern (VOCs) in vaccinated RMs. Besides, before progressing B/HPIV3/S-6P to a Phase I clinical trial, the team evaluated the protective ability of B/HPIV3/S-6P against the SARS-CoV-2 challenge.
Results and conclusions
According to the study results, following vaccination, B/HPIV3/S-6P and B/HPIV3 did not influence the overall health of RMs, showing that the expressed SARS-CoV-2 S protein and this vector were safe in this non-human primate species. A single intratracheal/intranasal B/HPIV3/S-6P vaccine dose generated robust SARS-CoV-2 S-specific mucosal immunoglobulin G (IgG) and IgA responses in the airway. The vaccination also induced high serum SARS-CoV-2 receptor-binding domain (RBD) and S-specific antibody titers (IgA, IgM, and IgG), effectively neutralizing the SARS-CoV-2 VOCs. The anti-RBD and anti-S IgG responses were equivalent to those found in the human convalescent plasma with high anti-RBD and anti-S IgG antibody titers.
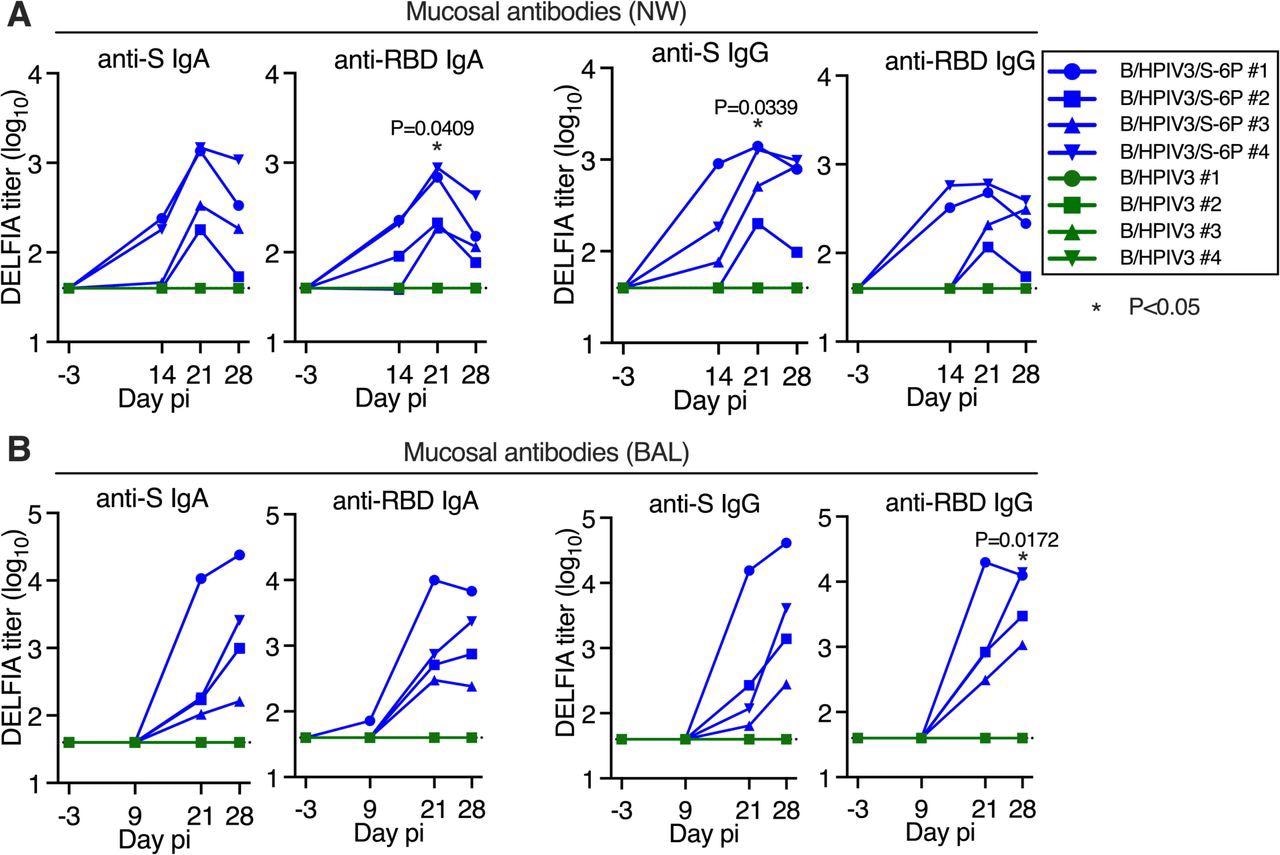
Intranasal/intratracheal immunization with B/HPIV3/S-6P induces mucosal antibody responses to SARS-CoV-2 S in the upper and lower airways. Rhesus macaques (n=4 per group) were immunized with B/HPIV3/S-6P or B/HPIV3 (control) by the intranasal/intratracheal route (Figure S1). To determine the mucosal antibody response in the upper airways, nasal washes (NW) were performed before immunization and on days 14, 21, and 28. To analyze the antibody response in the lower airways, bronchoalveolar lavages (BAL) were collected before immunization and on days 9, 21, and 28 pi. (A and B) S- and receptor binding domain (RBD)-specific mucosal IgA and IgG titers on indicated days post-immunization (pi) in the upper (A) and lower (B) airways, determined by time-resolved dissociation-enhance lanthanide fluorescence (DELFIA-TRF) immunoassay. Endpoint titers are expressed in log10 for mucosal IgA and IgG to a secreted prefusion-stabilized form (aa 1-1,208; S-2P) of the S protein (left panels) or to a fragment of the S protein (aa 328-531) containing SARS-CoV-2 RBD (right panels). The limit of detection is 1.6 log10 (dotted line). B/HPIV3/S-6P-immunized RMs are shown in blue, while B/HPIV3-immunized RMs are in green, with each RM represented by a symbol. *P<0.05 (two-way ANOVA, Sidak multiple comparison test).
The vaccine-matched SARS-CoV-2 WA1/2020 isolate and the Alpha and Delta VOCs were neutralized efficiently by serum antibodies. Nevertheless, these sera had minimal neutralizing efficacy against the Beta and Omicron VOCs. This inference showed that a booster dose of B/HPIV3/S-6P similar to any SARS-CoV-2 vaccination might be necessary to increase antibody concentrations and affinity maturation, allowing for a broader range of antigen identification and immunity against VOCs.
B/HPIV3/S-6P vaccination likely produced long-lived antigen-selective tissue-resident T and B cells in the mucosa, albeit this has not been confirmed. B/HPIV3/S-6P elicited strong pulmonary and systemic S-specific CD8+ and CD4+ T-cell responses, consisting of lung tissue-resident memory cells.
SARS-CoV-2 replication in lung tissues and airways of vaccinated macaques was undetectable after the viral challenge. RMs were completely protected against the SARS-CoV-2 challenge one month following vaccination. Under the present experimental circumstances, no SARS-CoV-2 challenge virus multiplication was detected in the airways, or lung tissues of vaccinated RMs, implying sterilizing immunity. On the other hand, the durability of protection was still unknown and will be investigated in a subsequent study. After the challenge, the authors found a recall reflex of S-specific CD4+ T-cells of B/HPIV3/S-6P-vaccinated RMs in the blood but not in the lower airway.
In summary, the study findings demonstrated that a single topical B/HPIV3/S-6P vaccination was significantly protective and immunogenic against COVID-19 in rhesus macaques. The current data encourage the development of this vaccine prospect as a solo vaccine or in a prime/boost mixture with existing SARS-CoV-2 vaccines for newborns and young children. A phase I trial will also be performed on B/HPIV3/S-6P, according to the authors.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Intranasal pediatric parainfluenza virus-vectored SARS-CoV-2 vaccine candidate is protective in macaques; Cyril Le Nouen, Christine E Nelson, Xueqiao Liu, Hong-Su Park, Yumiko Matsuoka, Cindy Luongo, Celia Santos, Lijuan Yang, Richard Herbert, Ashley Castens, Ian N Moore, Temeri Wilder-Kofie, Rashida Moore, April Walker, Peng Zhang, Paolo Lusso, Reed F Johnson, Nicole L Garza, Laura E Via, Shirin Munir, Daniel Barber, Ursula J Buchholz. bioRxiv preprint 2022. DOI: https://doi.org/10.1101/2022.05.21.492923, https://www.biorxiv.org/content/10.1101/2022.05.21.492923v1
- Peer reviewed and published scientific report.
Nouën, Cyril Le, Christine E. Nelson, Xueqiao Liu, Hong-Su Park, Yumiko Matsuoka, Cindy Luongo, Celia Santos, et al. 2022. “Intranasal Pediatric Parainfluenza Virus-Vectored SARS-CoV-2 Vaccine Is Protective in Monkeys.” Cell 185 (25): 4811-4825.e17. https://doi.org/10.1016/j.cell.2022.11.006. https://www.cell.com/cell/fulltext/S0092-8674(22)01418-0.