Studies have not examined the long-term impacts of prior exposure, vaccine regimen, and type at the level of T cell responses. In the absence of persistent immune protection conferred by T-cells, COVID-19 could progress to a severe form resulting in a patient’s death.
Therefore, it is essential to characterize the generation and maintenance of antibody and T cell responses against SARS-CoV-2 after both infection and vaccination, alone or in combination. Further, these insights could help determine future vaccination policies for those more vulnerable to prioritize them for extra booster shots and monoclonal antibody treatments.
About the study
In the present study, researchers characterized the long-term adaptive and humoral immune responses among HCWs who had received three doses of a COVID-19 vaccine, with a special focus on the impact of vaccine regimen, type, and prior infection.
They reported robust immunity to SARS-CoV-2 spike (S), including the Omicron BA.1 variant for three vaccine regimens. The team analyzed the response to BNT162b2 vaccination with a short and long dosing interval and two doses of the AZ1222 vaccine following boosting with BNT162b2.
The team sampled the eligible HCWs between 4 January 2021 and 15 February 2022, before the emergence of the Omicron BA.1 sub-variant in the United Kingdom (UK). They recorded the clinical characteristics, including BNT162b2 and AZD1222 vaccination dates, date of prior SARS-CoV-2 infection, the time between symptom onset and sampling, age, gender, and ethnicity of the eligible participants.
Further, the team randomly selected 95 HCWs from all three vaccine regimens with previous SARS-CoV-2 infection. They obtained their serum samples and used intracellular cytokine staining (ICS) to characterize their T cell responses. From this subset, they selected 73 individuals, with 27 participants from the BNT162b2 long and short interval groups and 19 participants from the AZD1222 group. Finally, they subjected their samples to a T cell proliferation assay to assess the magnitude of memory responses to SARS-CoV-2 S, membrane (M), and nucleocapsid (N) protein in the cluster of differentiation (CD)4+ and CD8+ T cell pool.
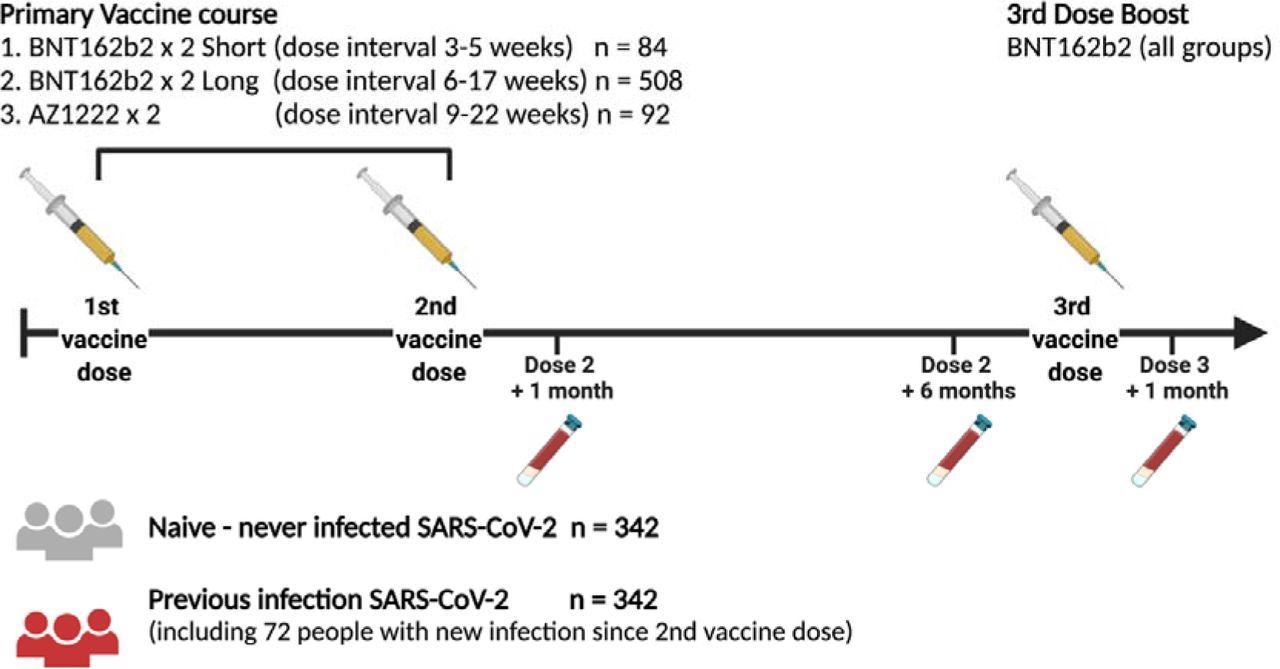
Study Design Schematic representation of vaccination and phlebotomy time points. Figure created using Biorender.
Study findings
The findings were consistent with a previous, more extensive SARS-CoV-2 immunity and reinfection evaluation (SIREN) study. Moreover, the authors observed no decline in the T cell responses over time regardless of the vaccine regimen. However, the booster shot decreased the impact of prior infection on the binding antibody responses.
The dynamics of BNT162b2 booster-induced immune responses varied across SARS-CoV-2 variants. While binding and neutralizing antibodies waned within the six months after the second dose, B and T cell ELISpot responses waned much less during the same time. Post-six months from the second vaccine dose, T cells proliferated and secreted multiple cytokines, indicating they retained a broad range of memory functions.
Nevertheless, the booster shot enhanced all immune responses, although the boost of T cell response was much smaller. Additionally, the booster shot greatly enhanced the neutralizing capacity of the antibody response. The findings validated that while a booster shot does not change the overall binding antibody levels, it drastically improves the quality of the antibody responses.
In the previously infected individuals, a booster shot induced a detectable T cell response and helped preserve vaccine effectiveness against hospitalization. Compared to antibodies, T cells have a wider range of available epitopes, so their responses are much less impacted by emerging SARS-CoV-2 variants.
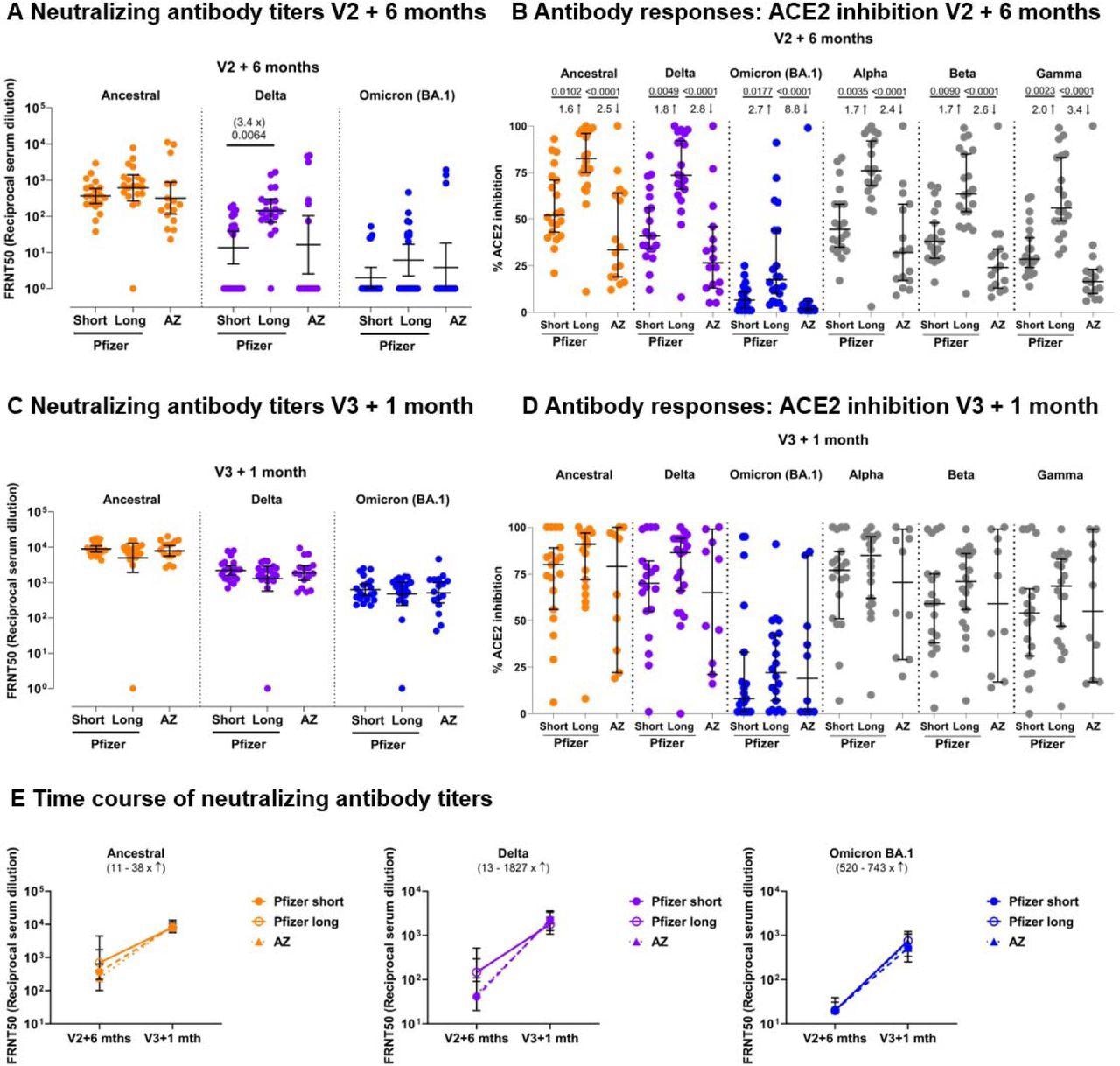
Neutralizing antibody titer profiles against SARS-CoV-2 variants of concern 6 months after 2 doses of BNT162b2 or AZ1222 and 1 month after a third vaccine with BNT162b2. Neutralizing antibodies against the Victoria isolate (orange), delta (B.1.617.2, purple) and omicron BA.1 (B.1.1.529 BA.1, blue) taken from infection-naïve participants after receiving 2 doses of BNT162b2 (Pfizer-BioNTech) vaccine delivered in a short (“Short”, 3-5 weeks, n=20) or long (“Long”, 6-17 weeks, n=20) dosing interval, or 2 doses of AZD1222 (AstraZeneca) vaccine (“AZ”, n=16) are shown in (4A) 6 months after the second dose, and (4B) for the same individuals, 1 month after a third “booster” dose with BNT162b2 for all participants. Geometric mean neutralizing titers with 95% confidence intervals are shown. Focus Reduction Neutralization Assay 50 (FRNT50) is the reciprocal dilution of the concentration of serum required to produce a 50% reduction in infectious focus forming units of virus in Vero cells (ATCC CCL- 81). (4C) Comparison of the data from (4A) and (4B), plotted as means with error bars by vaccine regimen. V2+6 months = 6 months after the second vaccine, V3+1 month = 1 month after the third “booster” BNT162b2 vaccine. The range of fold change (median) between V2+6 months and V3+1 month for the three vaccine regimens (Short – dashed line, Long – solid line, and AZ – dotted line) is shown in brackets for each variant. Data in (4A), (4B) and (4C) from the Short group (n=20) has been previously published (Dejnirattisai, Huo et al. 2022). (4D) Impact of Short or Long BNT162b2 vaccine dosing interval and AZ on the ability of sera to inhibit ACE2 binding to SARS-CoV-2 spike (Victoria isolate, delta (B.1.617.2), Omicron BA.1 (B.1.1.529 BA.1), alpha (B.1.1.7), beta (B.1.351) and gamma (P.1) 6 months after the second dose and (4E) 1 month after a third “booster” dose with BNT162b2. ACE2 inhibition was analysed using a multiplexed MSD® assay. Data are shown in percentage of inhibition. Bars represent the median with 95% confidence intervals. Naïve, Short: n=20; Naïve, Long: n=20; Naïve, AZ: n=16 for V2 + 6 months; Naïve, Short: n=19; Naïve, Long: n=20; Naïve, AZ: n=10 for V3 + 1 month. Vaccine regimens were compared with the Kruskal-Wallis nonparametric test and Dunn’s multiple comparisons tests, with 2-tailed p values shown above linking lines when 2- tailed p<0.05, and fold changes are shown between the columns.
Conclusions
Regardless of vaccine type and regimens, COVID-19 vaccination induced a stable pool of T cell memory that protected against severe disease in individuals with or without prior infection. Moreover, a booster shot conferred additional protection beyond the primary vaccination. It helped mount a more robust immune response to the Omicron BA.1 variant by inducing better antibody recognition and potentially reducing hospitalization rates in people with pre-existing comorbidities. Accordingly, several countries, such as the UK, are vaccinating their immunocompromised populations with fourth or even fifth vaccine doses.
Overall, the study provides a much-needed glimpse into evolving hybrid immunity driven by exposure to newer SARS-CoV-2 variants in vaccinated populations. Further studies should continue to investigate the role of booster vaccinations in more vulnerable populations (e.g., HCWs) to mitigate nosocomial SARS-CoV-2 transmission and the risks of Long COVID.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Evolution of long-term hybrid immunity in healthcare workers after different COVID-19 vaccination regimens: a longitudinal observational cohort study, Shona C Moore, Barbara Kronsteiner, Stephanie Longet, Sandra Adele, Alexandra S Deeks, Chang Liu, Wanwisa Dejnirattisai, Laura Silva Reyes, Naomi Meardon, Sian Faustini, Saly Al-Taei, Tom Tipton, Luisa M Hering, Adrienn Angyal, Rebecca Brown, Alexander R Nicols, Susan L Dobson, Piyada Supasa, Aekkachai Tuekprakhon, Andrew Cross, Jessica K Tyerman, Hailey Hornsby, Irina Grouneva, Megan Plowright, Peijun Zhang, Thomas A.H. Newman, Jeremy M. Nell, Priyanka Abraham, Mohammad Ali, Tom Malone, Isabel Neale, Eloise Phillips, Joseph D. Wilson, Adrian Shields, Emily C. Horner, Lucy H. Booth, Lizzie Stafford, Sagida Bibi, Daniel G. Wootton, Alexander J. Mentzer, Christopher P. Conlon, Katie Jeffery, Philippa C. Matthews, Andrew J. Pollard, Anthony Brown, Sarah L. Rowland-Jones, Juthathip Mongkolsapaya, Rebecca P. Payne, Christina Dold, Teresa Lambe, James E.D. Thaventhiran, Gavin Screaton, Eleanor Barnes, Susan Hopkins, Victoria Hall, Christopher JA Duncan, Alex Richter Richter, Miles Carroll, Thushan I. de Silva, Paul Klenerman, Susanna Dunachie, Lance Turtle, medRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.06.06.22275865, https://www.medrxiv.org/content/10.1101/2022.06.06.22275865v1
- Peer reviewed and published scientific report.
Moore, Shona C., Barbara Kronsteiner, Stephanie Longet, Sandra Adele, Alexandra S. Deeks, Chang Liu, Wanwisa Dejnirattisai, et al. 2023. “Evolution of Long-Term Vaccine-Induced and Hybrid Immunity in Healthcare Workers after Different COVID-19 Vaccine Regimens.” Med 4 (3): 191-215.e9. https://doi.org/10.1016/j.medj.2023.02.004. https://www.cell.com/med/fulltext/S2666-6340(23)00064-8.