Herein, the researchers observe that symptomatic Delta breakthrough infections have similar efficacy as a third booster vaccine dose in inducing cross-neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants.
Study: SARS-CoV-2 breakthrough infection during the Delta-dominant epidemic and neutralizing antibodies against Omicron in comparison with the third dose of BNT162b2: a matched analysis. Image Credit: MK photograp55 / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
Current COVID-19 vaccines are associated with high efficacy in reducing the risk of SARS-CoV-2 infection, symptomatic COVID-19, hospitalization, and mortality. However, due to declining vaccine efficacy with time and the emergence of SARS-CoV-2 variants with high immune evasion capabilities, millions of breakthrough infections have been reported throughout the world. As a result, a third booster dose of COVID-19 vaccines has been recommended to maintain protection against these variants.
Previous reports suggest that anti-SARS-CoV-2 immune responses in individuals who have received two COVID-19 vaccine doses and experienced Delta breakthrough infection are similar to those observed in people who have received three vaccine doses.
About the study
The current study included healthcare workers of the National Center for Global Health and Medicine in Japan. All participants attended a baseline serological survey in June 2021 and a follow-up survey in December 2021.
A total of 836 infection-naïve participants were identified who had received two doses of the messenger ribonucleic acid (mRNA) vaccine BNT162b2 (Pfizer/BioNTech) at the baseline. Of them, 11 were identified to have Delta breakthrough infection at the follow-up.
In the current study, the scientists assess the anti-wildtype SARS-CoV-2, anti-Delta, and anti-Omicron neutralizing efficacy of antibodies in two-dose vaccinated individuals who have been infected with the Delta variant after vaccination. This neutralizing efficacy was then compared to that which was observed in infection-naïve individuals with or without booster vaccination (third dose). For each case, infection-naïve controls were randomly selected from participants with or without third booster vaccination.
Important observations
All study participants had comparable SARS-CoV-2 spike-binding antibodies at baseline. The highest level of neutralizing antibodies was observed against the wild-type SARS-CoV-2 strain, followed by the Delta variant. None of the participants had detectable neutralizing titers against the Omicron variant at baseline.
Neutralizing titers against SARS-CoV-2 strains
At follow-up, a 4.1- and 10.9-fold induction in neutralizing titers against the wildtype SARS-CoV-2 strain were observed among participants who experienced Delta breakthrough infection or received the booster vaccination, respectively. In contrast, neutralizing titers became undetectable in infection-naïve participants who received two vaccine doses.
Regarding neutralizing titers against the Delta variant at follow-up, a 5.5- and 13.8-fold induction was observed among participants with breakthrough infection and those with booster vaccination, respectively. In contrast, a 2.8-fold reduction in Delta-neutralizing titers was observed in infection-naïve participants who had received two vaccine doses.
All study participants who had received a booster vaccine, as well as two-thirds of those with breakthrough infection, exhibited detectable titers against the SARS-CoV-2 Omicron variant at follow-up.
About three-fold reduced anti-SARS-CoV-2 spike antibody titers were observed in participants who experienced a breakthrough infection with the SARS-CoV-2 Delta variant as compared to the levels observed in boosted participants.
Symptomatic vs. asymptomatic infection
Participants who reported symptomatic breakthrough infections exhibited a 3.8- to 17.7-fold induction in neutralizing titers against the wildtype, Delta, and Omicron strains at follow-up. In contrast, participants with asymptomatic breakthrough infections exhibited a 3.1-fold reduction in anti-wildtype titers and no induction in anti-Delta and anti-Omicron titers at follow-up.
Omicron vs. Delta neutralizing titers
Considering all participants, 85% and 89% had detectable neutralizing titers against the wildtype and Delta strains, respectively, at baseline. In contrast, anti-Omicron neutralizing titers were undetectable in all participants. Overall, anti-Delta neutralizing titers were 1.6-fold lower than anti-wildtype titers.
As compared to anti-wildtype and anti-Delta neutralizing titers, anti-Omicron titers were significantly lower in participants with breakthrough Delta infection and those who received a booster vaccine dose.
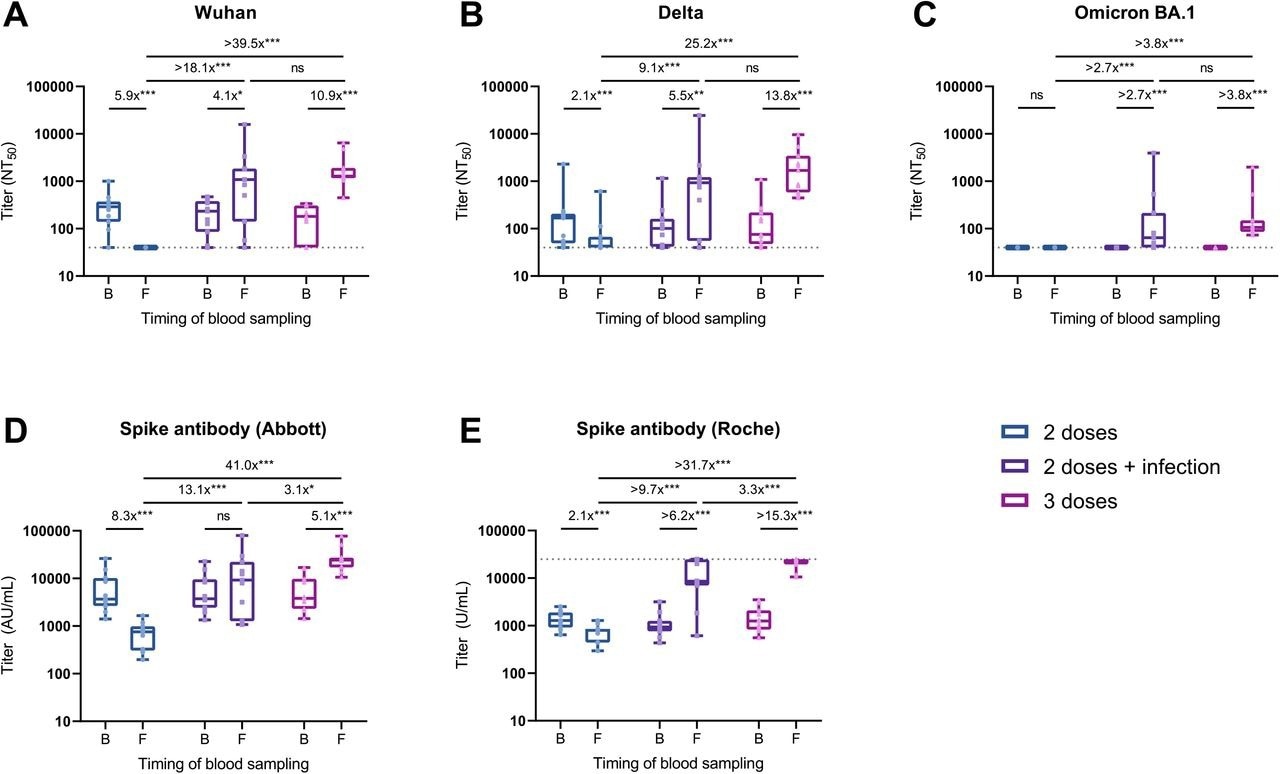
Change in the neutralizing and spike antibody titers in individuals who experienced breakthrough infection, received the booster vaccine, or were unboosted during follow-up. Shown are NAb titers against the original Wuhan strain (A), the Delta variant (B), and the Omicron BA.1 variant (C) determined by 50% focus reduction neutralization test (FRNT50) using the serum at baseline and follow-up. Also shown are anti-spike antibody titers measured with the Abbott reagent (D) and the Roche reagent (E) at baseline and follow-up. Box plots show the median, interquartile range, and full range. The dushed horizontal lines indicate the LOD in the present analysis (NT50<40 in FRNT50 and U/mL>25000 in Roche assay).
The fold-change values are estimated ratios of geometric means for antibody titers based on the GEE model (ns: not significant; *P<0.05; **P<0.01; ***P<0.001). Abbreviations: AU, arbitrary units; B, baseline; F, follow-up; GEE, generalized estimating equation; LOD, limits of detection; NT50, 50% neutralization titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
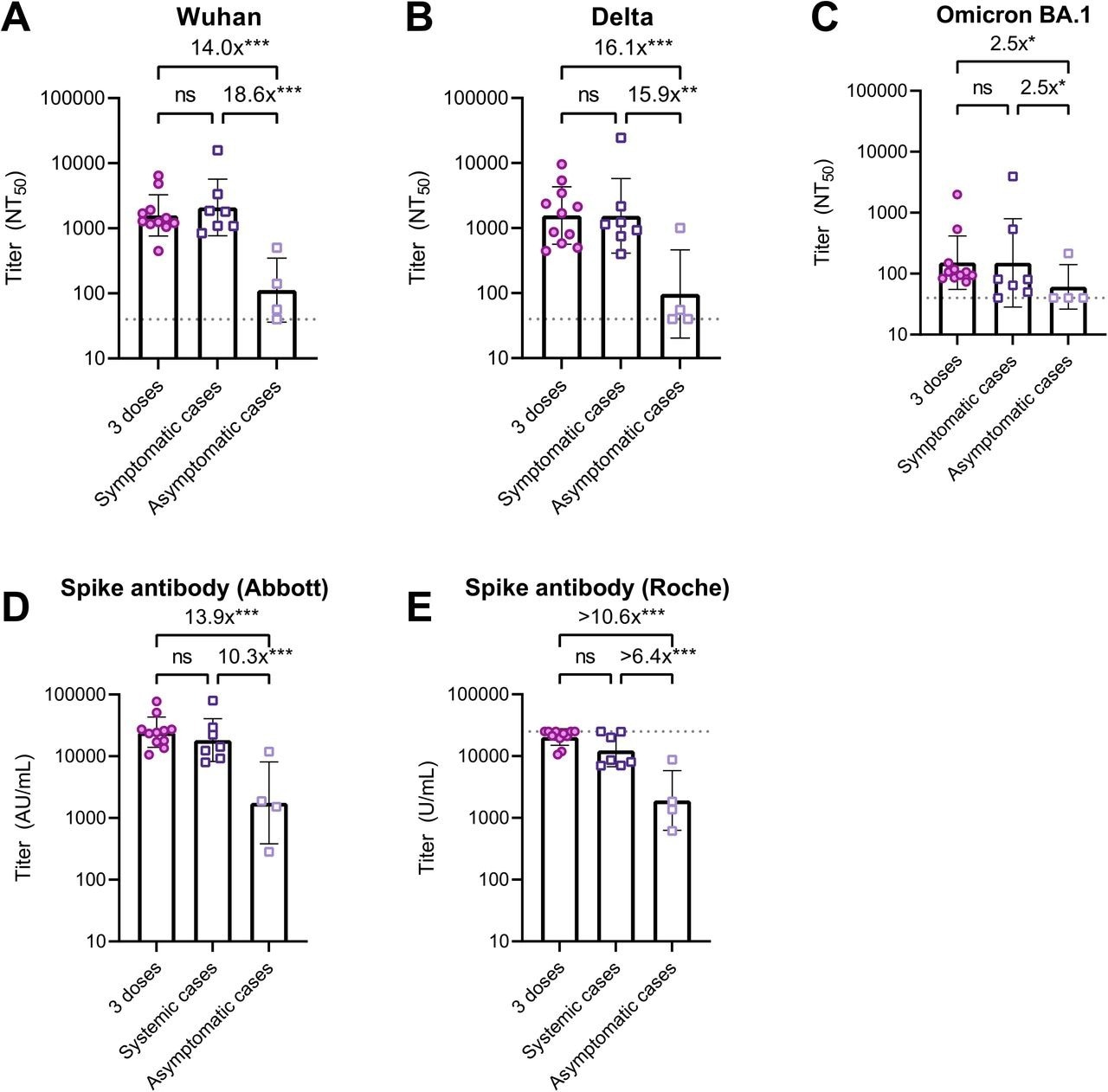
Neutralizing and spike antibody titers after three vaccine doses, symptomatic and asymptomatic breakthrough infections. Shown are NAb titers against the original Wuhan strain (A), the Delta variant (B), and the Omicron BA.1 variant (C) determined by 50% focus reduction neutralization test (FRNT50) using the serum at follow-up. Also shown are anti-spike antibody titers measured with the Abbott reagent (D) and the Roche reagent (E) using the serum at follow-up.
Patients with PCR-confirmed infection were all symptomatic (n=7), while those with seropositive on any of anti-SARS-CoV-2 nucleocapsid protein assays (Abbott or Roche assays) at follow-up were all asymptomatic (n=4). The bars indicate geometric mean titers, and I-shaped bars indicate its geometric standard deviations. The dushed horizontal lines indicate the LOD for FRNT50 (NT50<40) and Roche assay (U/mL>25000) in the present analysis. Statistical significance was determined by Kruskal-Wallis and Dunn’s multiple comparison test (ns: not significant; *P<0.05; **P<0.01; ***P<0.001).
Abbreviations: AU, arbitrary units; COVID-19, coronavirus disease 2019; LOD, limits of detection; NT50, 50% neutralization titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Implications
The current study highlights that both symptomatic Delta breakthrough infection and a three-dose COVID-19 vaccination regimen have equivalent efficacy in inducing neutralizing antibody titers against the wildtype SARS-CoV-2 strain, as well as both the Delta and Omicron variants.
Considering the much lower neutralizing titers against immune evasive variants like Omicron, the scientists recommend the implementation of infection-controlling measures, irrespective of the population-level vaccination coverage.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.