In a recent study posted to the medRxiv* pre-print server, Canadian researchers evaluated antibody responses in kidney transplant recipients (KTRs) before and one and three months after receiving the third dose of a messenger ribonucleic acid (mRNA)-based coronavirus disease 2019 (COVID-19) vaccine.
 Study: Humoral Responses in the Omicron Era following Three-Dose SARS-CoV-2 Vaccine Series in Kidney Transplant Recipients. Image Credit: Corona Borealis Studio / Shutterstock
Study: Humoral Responses in the Omicron Era following Three-Dose SARS-CoV-2 Vaccine Series in Kidney Transplant Recipients. Image Credit: Corona Borealis Studio / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
KTRs, due to their suppressed immunity, mount weaker antibody responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination. A deeper understanding of immune responses in KTRs following a booster shot could help devise treatment strategies for those unprotected against SARS-CoV-2's most recent variant of concern (VOC) Omicron. Previous studies have reported diminished responses in KTRs relative to other organ transplant groups; however, data on the durability of the immune response beyond one month in KTRs is lacking.
About the study
In the present study, researchers collected blood samples from 44 KTRs before the administration of the third dose of the COVID-19 vaccine and after one and three months post-vaccination. All these patients were under treatment with a calcineurin inhibitor (CNI), with 91% of them taking tacrolimus. The median age of the study cohort was 55.5 years, with around 80% male patients.
All KTRs had received a two-dose regimen of an m-RNA-based COVID-19 vaccine; 35 and eight had received BNT162b2 and mRNA-1273, respectively. At the time of taking a booster shot or third dose of a COVID-19 vaccine, 70.5% of KTRs were on a standard immunosuppressive regimen. The researchers could analyze only 26 of 44 KTRs at three months post-third dose. The control group comprised 13 healthy individuals with a mean age of 46 years, and they had received two doses of the BNT162b2 vaccine. The researchers measured the anti-receptor-binding domain (RBD), anti-spike (S), and anti-nucleocapsid immunoglobulin G (IgG) levels in the plasma of all KTRs who had received the third dose of the SARS-CoV-2 vaccine.
Study findings
Most KTRs had detectable anti-S and anti-RBD antibodies one and three months after receiving a booster dose. Those who exhibited a robust immune response at one month also had a preserved humoral response till three months. However, the immune response of KTRs was inferior to that observed in healthy controls. The study results also determined anti-RBD and anti-S antibody thresholds that could point out patients with no neutralizing antibodies against Omicron. In fact, an Omicron-specific neutralizing response among KTRs was limited, with neutralizing antibodies to Omicron in only around 45% of the cohort population. Moreover, only 38.5% had a sustained response against Omicron after three months following an mRNA vaccine booster dose.
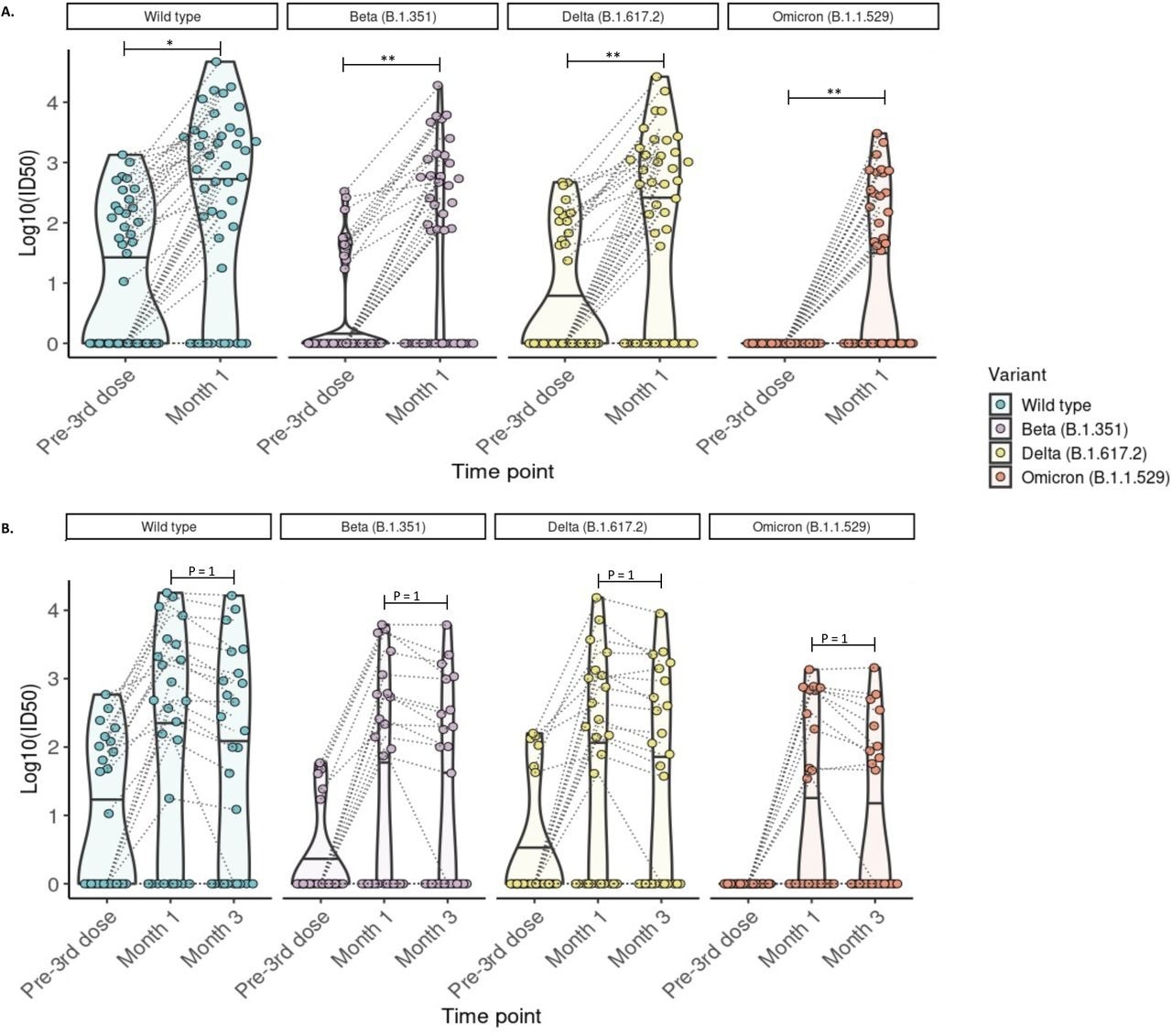
Detection of neutralizing antibodies against SARS-CoV-2 wild-type, Beta, Delta, and Omicron (BA.1) variants at 1 and 3 months post-third mRNA vaccine dose. A) Neutralizing antibodies were detected in 44 participants with blood samples drawn pre- and at 1-month post-third dose. The proportion of kidney transplant recipients with detectable neutralizing antibody (Log10ID50 >0) was significantly increased for all variants (McNemar test with continuity correction; wild-type p =0.026, Beta p= 0.005, Delta p = 0.005; McNemar’s exact test: Omicron p=0.0037). B) Neutralizing antibodies were detected in 26 participants with blood samples drawn pre- and at 1 and 3 months post-third dose. The proportion of kidney transplant recipients with detectable neutralizing antibodies (Log10ID50 >0) was not significantly altered compared to month 1 (McNemar test with continuity correction; p=1 for all comparisons. For all images: Paired values are linked with black dashed lines. Solid black lines in each violin plot indicate the median Log10ID50 values for each variant. * p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Preliminary studies have shown that a fourth vaccine dose does not help mount an immune response in those with a poor response to a three-dose vaccine series. Accordingly, the titers of binding and neutralizing antibodies against Omicron remained largely unchanged in solid organ transplantation recipients (SOTRs). Even in the current study cohort, only 10% of previous non-responding KTRs after three doses attained an adequate immune response following a fourth booster dose. This rationalizes why it is crucial to identify the subset of vaccinated transplant recipients who will remain at high risk despite booster vaccinations.
Furthermore, studies have proved that demographic and transplant-related clinical characteristics of SOTRs are significantly associated with the presence of an Omicron-specific neutralization response at one month post-third dose. The authors also noted that KTRs with a lower average age and transplant vintage in the study cohort were more likely to have detectable Omicron neutralizing antibodies. Additionally, they had a higher estimated glomerular filtration rate (eGFR). Although not statistically significant, a greater dosing interval between the second and third vaccine dose also impacted neutralizing antibody titers against at least one SARS-CoV-2 variant in KTRs.
Conclusions
The binding and neutralizing antibody titers directly correlate with protection from SARS-CoV-2 infection. Since the binding antibody assays are readily scalable and have high throughput, these seem to be the easiest and best means of identifying the subset of transplant patients unprotected against SARS-CoV-2 infection despite having received booster vaccines.
Further, determining the binding antibody thresholds for KTRs could aid risk-stratification of patients and identification of those requiring additional treatment. However, re-defining these parameters in the setting of each new SARS-CoV-2 variant and associated degree of immune evasion is also needed. This would enable personalized care for transplant recipients, including KTRs, targeted allocation of therapeutic monoclonal antibodies, revamped vaccination strategies, or novel vaccines for this high-risk population.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Humoral Responses in the Omicron Era following Three-Dose SARS-CoV-2 Vaccine Series in Kidney Transplant Recipients, Caitriona M. McEvoy, Queenie Hu, Kento T. Abe, Kevin Yau, Matthew J. Oliver, Adeera Levin, Anne-Claude M. Gingras, Michelle Hladunewich, Darren Yuen, medRxiv pre-print 2022, DOI: https://doi.org/10.1101/2022.06.24.22276144, https://www.medrxiv.org/content/10.1101/2022.06.24.22276144v1
- Peer reviewed and published scientific report.
McEvoyCaitríona M., Queenie Hu, Kento T. Abe, Kevin Yau, Matthew J. Oliver, Adeera Levin, Anne-Claude Gingras, Michelle A. Hladunewich, and Darren A. Yuen. 2023. “Humoral Responses in the Omicron Era Following 3-Dose SARS-CoV-2 Vaccine Series in Kidney Transplant Recipients.” Transplantation Direct 9 (1): e1401. https://doi.org/10.1097/TXD.0000000000001401. https://journals.lww.com/transplantationdirect/Fulltext/2023/01000/Humoral_Responses_in_the_Omicron_Era_Following.2.aspx.