Well into the third year of the coronavirus disease 2019 (COVID-19) pandemic, novel vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continue to be developed. However, many groups and individuals remain hesitant about COVID-19 vaccination, including pregnant women who are at high risk for severe disease and adverse pregnancy outcomes.
Study: Cross-Sectional Survey of High-Risk Pregnant Women’sOpinions on COVID-19 Vaccination. Image Credit: Andrey_Popov / Shutterstock.com
Introduction
The COVID-19 pandemic led to extensive and crippling waves of disease worldwide. This triggered the implementation of quarantine measures that ceased travel, business, education, and social interactions.
The resulting disruption to global life led to an intensive effort by many pharmaceutical companies to develop safe and effective vaccines against SARS-CoV-2. The first type of COVID-19 vaccine to gain approval was based on the messenger ribonucleic acid (mRNA) platform. Since then, several billion mRNA vaccine doses have been administered throughout the world.
Pregnant women were not included in the clinical trials of mRNA vaccines and were initially hesitant to receive these vaccines for fear of their impact on their health and that of their babies. This fear has been cited, even though COVID-19 during pregnancy is associated with a greater risk of severe disease, mechanical ventilation, and death as compared with non-pregnant women.
Pregnant women who were at high risk for COVID-19, such as frontline medical and other workers, received COVID-19 vaccines early on in the pandemic. Observational studies have shown that no harm was caused to the mother nor fetus as a result of these vaccinations.
This success has been referenced by many professional societies and public health agencies like the United States Centers for Disease Control and Prevention (CDC) to demonstrate that mRNA COVID-19 vaccines are safe during pregnancy.
Despite the recommendations of these bodies, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal Fetal Medicine (SMFM), and the CDC, social media pressure and the lack of clinical trial data have led many pregnant women to still resist vaccination.
Study findings
About 160 women participated in the current study, only about 33% of whom had received the vaccine. Of those who had not yet been vaccinated against COVID-19, most cited lack of information about the vaccine used in pregnancy as the reason for their unvaccinated status and feared adverse effects.
Over 50% of study participants indicated that they felt the vaccine platform was too novel for them to trust its safety. About a third did not trust the government’s benevolent intentions in recommending widespread vaccination.
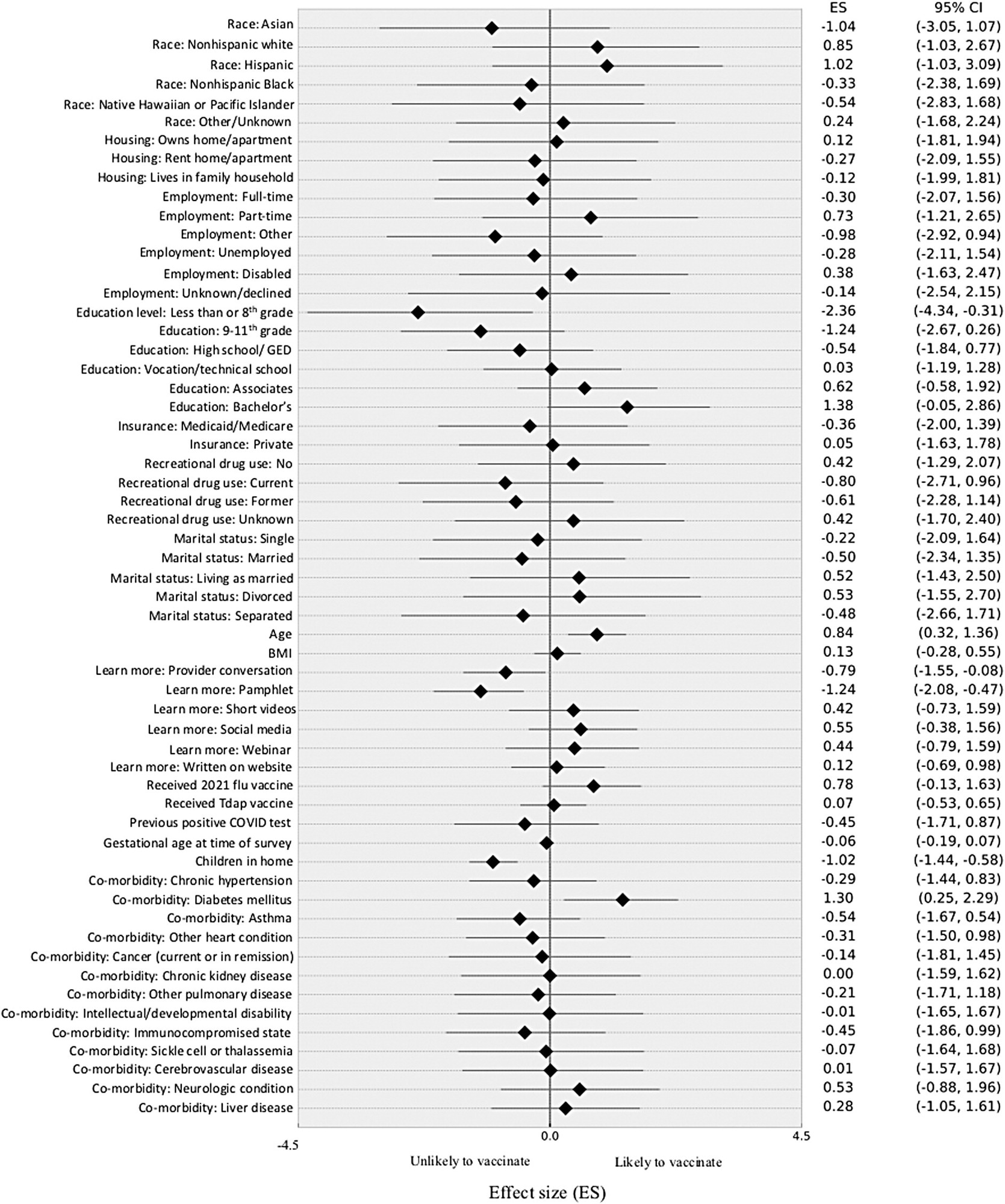
There was a weak positive trend with the level of education, with those who had completed less than eight years of formal schooling being less than half as likely to be vaccinated. The differences in the other groups, from those who have completed ninth grade to a bachelor’s degree, were not significant.
 Hierarchical logistic Bayesian regression.
Hierarchical logistic Bayesian regression.
Other factors did not appear to play a major role in the decision, including race, type of residence, employment, marital status, type of insurance, and drug use. The woman’s body habitus, number of children living, or term of pregnancy also did not have a significant difference on the decision to be vaccinated.
A history of influenza or tetanus-diphtheria pertussis (Tdap) vaccination also failed to show any correlation with COVID-19 acceptance rates. Interestingly, only a third of patients who received COVID-19 vaccines had taken the Tdap vaccine, whereas two-thirds of unvaccinated women had received this vaccine, though this difference failed to reach statistical significance.
Among study participants with high-risk factors such as hypertension, heart conditions, asthma, diabetes mellitus, cancer, chronic kidney disease, and developmental disabilities, only diabetes was associated with a 30% higher likelihood of COVID-19 vaccination.
Overall, the women said they would prefer to learn more about the vaccine from their physicians as compared to pamphlets, videos, websites, or social media. This response was referenced by half of the unvaccinated women as compared to 60% of vaccinated women. The least popular choice was a recorded short video or webinar and was selected by 10% or less of both groups.
Implications
Vaccination rates among pregnant women continue to be low. It appears that a lack of trustworthy data regarding the safety of current COVID-19 vaccines in pregnancy is the primary obstacle.
The disconnect between influenza and Tdap vaccination and COVID-19 vaccination status in pregnancy belies hypotheses that hesitation regarding COVID-19 vaccination reflects a negative view towards vaccines in general. The importance of discussing the COVID-19 vaccine with patients during elective antenatal visits is evident given the confidence women expressed in gaining information about this vaccine from their doctors.
Perhaps the slightly higher rate of vaccination among pregnant diabetic women is due to their increased frequency of interaction with their healthcare providers, through which they may have gained a positive view of the vaccine as a result of increased information.
Further research could involve providing more clinical visits and assessing any change in vaccine willingness thereafter.
COVID-19 vaccination opinions are dynamic throughout the natural history of the pandemic and future studies may be useful tore-evaluate patients’ evolving views.”
Journal reference:
- DesJardin, M., Raff, E., Baranco, N., et al. (2022). Cross-Sectional Survey of High-Risk Pregnant Women’sOpinions on COVID-19 Vaccination. Women’s Health Reports. doi:10.1089/whr.2022.0006.