As of July 20, 2022, SARS-CoV-2 has infected over 566 million individuals and caused almost 6.4 million deaths worldwide. Despite the success of global vaccination campaigns in reducing COVID-19-related mortality and morbidity, emerging viral variants and declining vaccine efficacy have perpetuated new infection waves.
Considering the sharp rise in vaccine breakthrough cases, many public health agencies have approved additional vaccine doses to the general population. These initiatives have been supported by growing evidence demonstrating that a third vaccine dose is highly effective in inducing a robust neutralizing antibody response, as well as the risk of severe COVID-19 and hospitalization.
In Israel, a third dose of the Pfizer/BioNTech mRNA-based COVID-19 vaccine was first introduced in August 2021 to control the spread of the SARS-CoV-2 Delta variant. Soon after, a fourth vaccine dose was introduced in January 2022 to combat the exponential rise in Omicron cases throughout the country. A fourth COVID-19 vaccine dose has been prioritized for immunocompromised patients, healthcare workers, and elderly people aged 60 years and above.
About the study
The current study included 608 healthcare workers from four different medical centers in Israel. Of all enrolled participants, 365 received three vaccine doses, and 243 received four vaccine doses.
During the 90-day follow-up period, the occurrence of SARS-CoV-2 infection, as well as vaccine-induced antibody responses, were determined at enrollment and at days 30 and 90 post-enrollment. The average follow-up days were 76 and 75 for the four-dose and three-dose groups, respectively.
Both immunoglobulin G (IgG)- and IgA-specific antibodies targeting multiple SARS-CoV-2 antigens were measured using blood samples collected from the participants.
Study findings
About 45% and 30% of participants from the three- and four-dose groups, respectively, developed SARS-CoV-2 infection during the 90-day follow-up period.
At day 30 post-enrollment, significantly higher titers of both IgG and IgA antibodies against the original strain of SARS-CoV-2 were observed in four-dose vaccinated participants compared to that at enrollment. Moreover, four-dose vaccinated participants showed significantly higher IgA antibodies against SARS-CoV-2 variants.
In three-dose vaccinated participants, a decline in IgG titers against the wild-type SARS-CoV-2 strain, as well as IgA and IgG titers against SARS-CoV-2 variants, was observed at day 30 post-enrollment.
A significant induction in neutralizing antibody titers against the wild-type, Delta, and Omicron strains was observed in four-dose vaccinated participants at day 30 post-enrollment.
In SARS-CoV-2-infected participants, a significant increase in antibody levels was observed at day 30, irrespective of the number of vaccine doses received. However, a reduction of about 37% in the risk of symptomatic infection was observed among participants who had received four vaccine doses.
The baseline IgA response to the wild-type SARS-CoV-2 strain, as well as its variants, showed the highest correlation with the risk of infection in four-dose vaccinated participants at day 30. Comparatively, the baseline IgG response to variants with mutations in the spike receptor-binding domain (RBD) showed a significant correlation with the risk of infection in three-dose vaccinated participants during the entire follow-up period.
Upon comparing four-dose vaccinated participants with low or high antibody levels after the third vaccine dose, a significantly higher number of infections were observed in participants with low antibody levels as compared to those with high antibody levels.
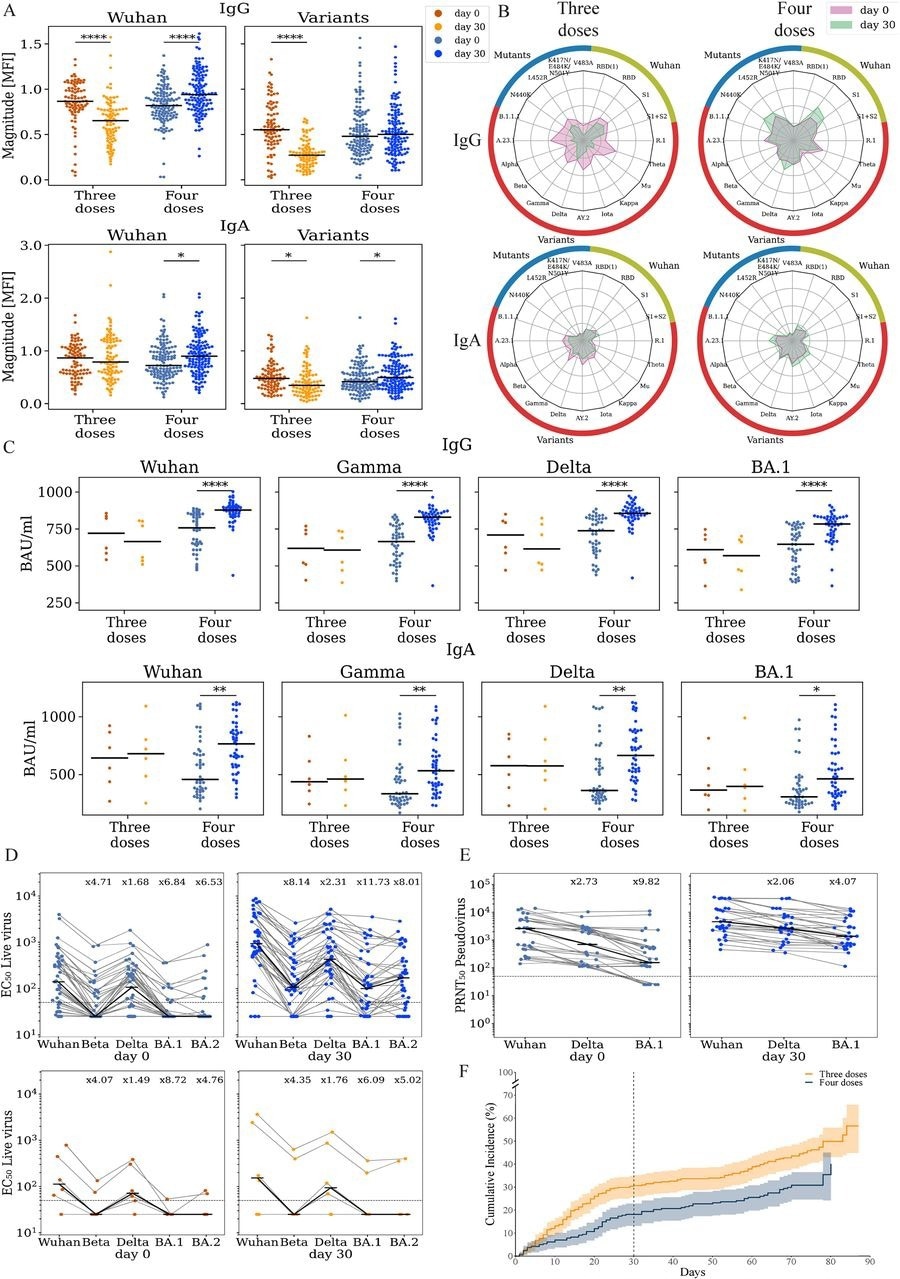
Vaccination with the 4th dose elicited binding and neutralizing antibodies against SARS-CoV-2. Responses of uninfected participants were analyzed at enrollment (day 0) and at day 30 using multiple serological assays (A) IgG and IgA magnitude to antigens from the Wuhan strain and SARS-COV-2 variants. Antigen microarrays spotted with RBD, S1 and spike proteins of the Wuhan vaccine strain and multiple other variants of concern were used to measure the magnitude of responses at day 0 (enrollment) and day 30 post-enrollment. (B) Spider plots depicting the enrollment (pink) and day 30 (green) antibody levels to Wuhan antigens (green), variants of concern (red) and RBD mutants (blue). The average normalized magnitude to each antigen is plotted in individuals that received 3 or 4 doses. (C) IgG and IgA anti-RBD ELISA binding titers for a subset of 74 participants (D) Live-virus neutralization EC50 titers of the same individuals in panel C. (E) Pseudovirus neutralization titers of uninfected individuals that received 4 (n=30, blue). (F) Cumulative incidence of SARS-CoV-2 infections in participants receiving three (n=365) vs. four doses (n=243) of the Pfizer vaccine. Four doses of the vaccine significantly reduced infection rates at day +30 (HR=0.55, p=0.002) and across all interim followup time (HR=0.63, p=0.003) as compared to three doses. * p < 0.05; ** p < 0.001; *** p < 0.0001; **** p < 0.00001.
Study significance
Combinations of IgA and IgG antibody levels were found to be responsible for providing protection against symptomatic SARS-CoV-2 infection following receipt of three and four doses of COVID-19 vaccines. Notably, a fourth vaccine dose was found to reduce the risk of symptomatic infection by 37%.
The current study also highlights that individuals with a low antibody response after the third vaccine dose are at a higher risk of developing symptomatic infection, despite receiving the fourth vaccine dose.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.