Sponsored Content by nanoComposixReviewed by Alex SmithJul 28 2022
In this interview, News-Medical talks to Philippe Riviere, Senior Scientist at nanoComposix (now a Fortis Life Sciences company) about mesoporous silica nanoparticles and the possibilities and opportunities they provide to drug delivery, vaccine development, and more.
What are nanoparticles, and how can they be used?
Nanoparticles are tiny objects. To give you an idea of the scale, we are talking about something that is 10-9 meters in size, the size of a nanoparticle relative to a football is the same as a football relative to the earth. These objects are very small, and their applications are various – and of course, they are around us in everyday life. For instance, they can be used as an anti-reflecting coating, in semiconductors, and, of course, they can be used in nanomedicine.
Could you please explain some of the applications of nanoparticles in which Fortis Life Sciences specializes?
At Fortis Life Sciences - we specialize in two main departments. The first of these is the analytical department. This is what we sometimes call the ‘lateral flow’ department. You may be familiar with these as, for example, the pregnancy test. These tests are based on gold nanoparticles, and that is the reason why they appear to the human eye as red most of the time. On the other side, we have nanomedicine applications and more treatment applications. We are trying to bring the advantages of nanoparticles primarily to treat diseases - mainly cancer- but it can be anything. We also have a branch that deals with viruses.
Image credit: nanoComposix - A Fortis Life Sciences Company
Why are nanoparticles so important in medicine?
Nanoparticles are so important in medicine because they are the ideal size to navigate inside the body: they can specifically target various sites or just go with the blood flow. Their size is a huge advantage compared to microscopic or drug molecules that have nonspecific activity because of their very small size in comparison.
How are nanoparticles used in drug delivery, and how would you say their use and this field has developed over the past 10 years or so?
The field is growing exponentially and has been for more than ten years: I would say it is probably more like 20 to 30 years in which we have seen this growth. Nanoparticles for drug delivery, in general, were developed based on polymeric material back in the 1970s. Today, more and more attention has arisen because of FDA approval for some of the materials involved, leading to many people becoming interested in this.
The real question is, why are nanoparticles so interesting in drug delivery? As I mentioned, typically, when you have just a drug molecule, and you try to absorb it or inject it, as it is so tiny, it will be taken up by pretty much anything in the body. This means that you need to have a large amount of it so that a small portion can actually traffic to the right organ or perform the specific action.
In the case of nanoparticles, you can design them so that they can target specific organs while preventing premature release and so avoid this inherent toxicity due to the very large amounts of the drug that have historically been needed.
What are mesoporous silica nanoparticles?
Mesoporous silica nanoparticles are a branch of nanoparticles and one of the nanoparticles used in drug delivery. There are two main components here, of which the first is silica. Silica is glass, in general, and this glass is made of silicon oxide. This silicon oxide is already present in your body in a very small amount. When you make nanoparticles with this, you, therefore, have the ability to easily modify their surface.
To briefly explain the ‘mesoporous’ part: this part of the name is actually telling you that your particle is not a plain or smooth particle, like a football. It is actually more like a golf ball: the surface rugosity means that holes have been drilled. This gives you a high amount of drug encapsulation capability because of the very high surface area in silica. This is what is meant by mesoporous silica nanoparticles.
What advantages would you say these mesoporous silica nanoparticles offer to nanomedicine versus other types of nanoparticles?
If you were to compare mesoporous silica nanoparticles to polymeric-based nanoparticles, for example, in terms of mass ratio, the amount of drug that you can load inside the mesoporous silica nanoparticles is much higher. Sometimes it can be up to an order of magnitude higher. Once again, this is very advantageous in terms of toxicity, as you would have to inject much less material to target the same kind of effect.
Other advantages include surface modification. Almost anything is possible with a silica in terms of chemistry. This will allow you to have a hydrophobic surface or if you want, a hydrophilic surface. It can have a positively charged or negatively charged surface. Once again, it can bring to the table the targeting ability I spoke about, so you can easily graft an antibody on the surface of the particle or another molecule that would specifically target a specific organ.
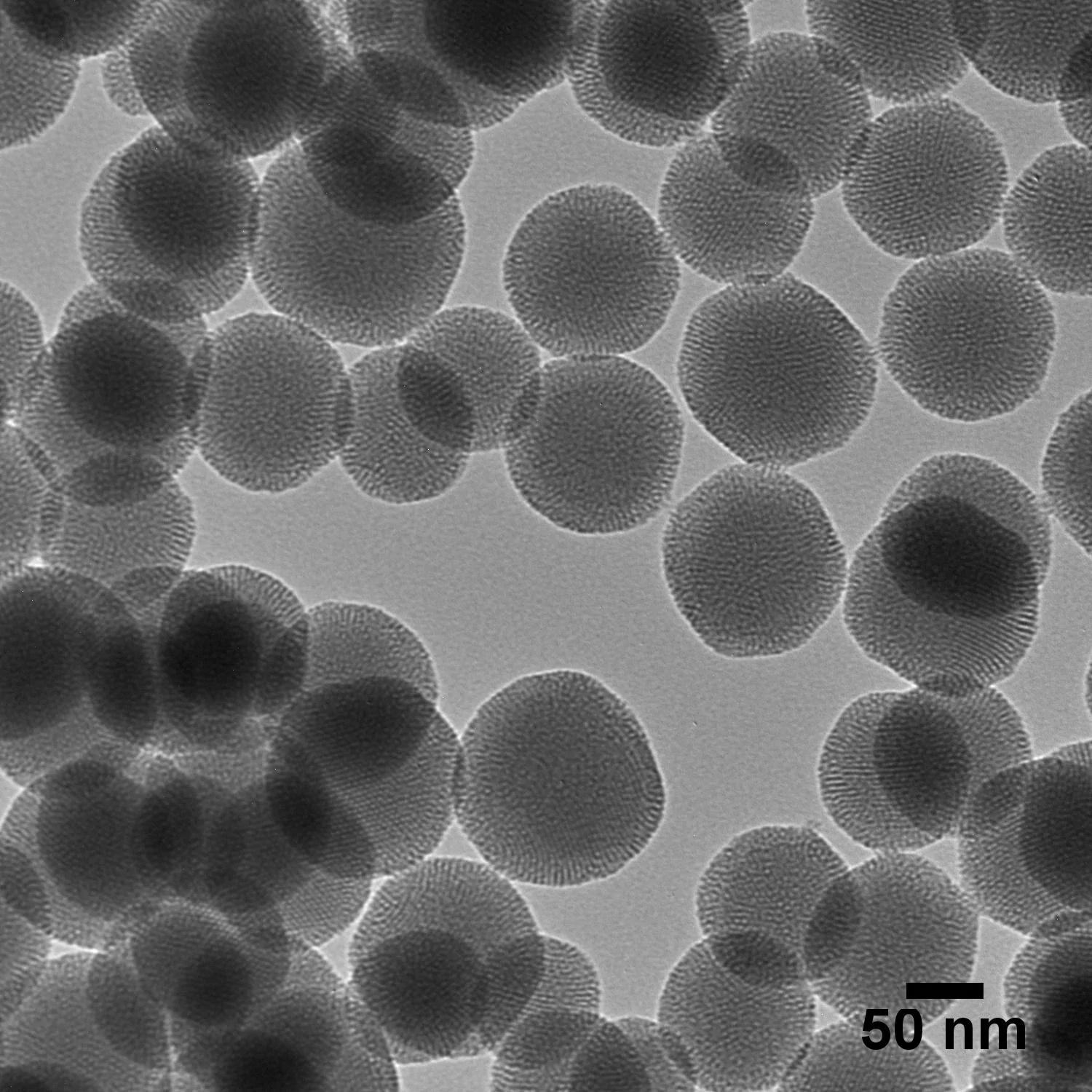
Image credit: nanoComposix - A Fortis Life Sciences Company
How would you say these particles could be utilized in gene therapy?
For gene therapy, which is another main advantage of these particles, there is a great utility because the user can control the pore size. As we touched on before, you can drill those holes in those golf balls – and therefore, you can actually define which size of holes you want in there. To accommodate any genetic material, you can expand the size of those holes and have all your genetic material completely capped inside this inorganic matrix. This will then be completely free of premature degradation upon injection into the body. This is the main advantage compared to polymeric material, which tends to degrade faster, and, again, would require a much higher dose in terms of treatment.
How can mesoporous silica nanoparticles be used in the development of vaccines?
I specifically worked on this using an NIH grant. My work focused on using mesoporous silica nanoparticles in the production of an HIV vaccine. There, the goal was to have a genetic material, so this was messenger RNA. Today, we are all much more familiar with this concept, thanks to COVID-19. We had message RNA that was coding for a specific protein of the HIV virus, and this was encapsulated inside the nanoparticles.
Then the next step was to have a targeting group. In our case, we used a molecule that was able to target dendritic cells. The dendritic cells are one of the main components in your immune-response system. These are what we call antigen-presenting cells. The fact that we have a target molecule on the surface of our silica allowed us to target specifically dendritic cells, and those dendritic cells will then traffic back into the lymph node and release the cargo. In this case, it is the silica that will then, in turn, release the RNA molecule - and this RNA molecule will be transfected and then translated into the cells to produce the protein in question.
This is an overview of how mesoporous silica are used in vaccine development. It can be used, as I say, for almost anything else. The most common application, arguably, is in cancer treatment, where the process is a little bit similar, but instead of targeting antigen-presenting cells, you are targeting the tumors directly.
What are the challenges of designing and manufacturing mesoporous silica nanoparticles? Are there any in particular that Fortis Life Sciences has encountered specifically?
The main challenges arise from the main advantages of the particles, confusingly enough. As I have stated, we can very precisely control the size of the material and the size of the pores.
When you go to scale and manufacturing, you need to think about reagents, the purity of reagents and what kind of reactor you are using to make the material. These particles are very finicky in terms of reaction conditions. So, more often than not, when you try to scale up, you will have to readjust every single parameter.
Every order of magnitude in terms of scaling up requires some optimization. I would say that this is the main challenge: it is extremely difficult to go from lab-scale all the way to pilot-scale in one go.
How does your team at Fortis Life Sciences in general, work with their clients to develop therapeutics that use these particles?
There are broadly two approaches. We have some clients that have a very defined project. All they will ask us is if this is doable with their material or not. In that case, we will just provide some input and, of course, all the expertise that we have in-house to help them succeed in their goal.
We also have another kind of project where clients already have something that they have developed; here, our expertise is to help them with the manufacturing of the same material. As we have this expertise - and because we tend to troubleshoot a lot of different nanoparticles and manufacture them at scale - we know exactly what hurdles they will encounter along the way. This is where we come into play for this kind of client.
Fortis Life Sciences has a multidisciplinary team of scientists with a vast range of background in chemistry, physics, engineering, and earth sciences. What are the main advantages you would say that this huge range of expertise would bring to your workflow and to any work that you conduct with your clients?
The way the team is built - at least, the research and development team - is that we have people with a range of skills. For instance, we have people that are specialists in chemistry; we have some with an engineering background; and others that have a biological background. These people are all working closely together, bringing different aspects to the projects.
This means that when we talk to clients, we do not approach the problem or the challenge that the client is facing from one angle, such as from only a chemistry angle or a biological angle. Given that we have those people who can think completely out of the box, we can bring a much wider view to the challenge and help the clients. The fact that we have people with different skills also showcases that we can help not only in the development of the material but also in the characterization of the material. It can be an in vitro study, or it can be an in vivo study. We can help design all of that thanks to the varied expertise of our team.
About Dr. Riviere
Philippe did his Ph.D. on sol-gel chemistry applied to the photooxidation of organic molecules. He then explored the synthesis of various inorganic nanoparticles (silica, silver, gold) as well as the antibacterial properties of core-shell materials at the University of Tokyo before specializing in the synthesis of mesoporous silica nanoparticles applied to drug delivery at the University of California, Los Angeles (UCLA). After joining nanoComposix, Philippe’s main research focuses on synthesis and derivatization of silica nanoparticles for applications in nanomedicine.

About nanoComposix (a Fortis Life Sciences Company)
Since 2004, nanoComposix has provided monodisperse and unagglomerated metal and metal-oxide nanomaterials to thousands of customers. Hundreds of different variants of material, size, shape, and surface are available as stock products and we have produced over 2000 custom core/shell, biofunctionalized, fluorescent, and magnetic nanocomposites to meet client specifications. nanoComposix produced the NIST nanosilver reference material and our particles have been utilized in over 2000 peer-reviewed publications. All of our materials are supplied with certificates of analysis that include electron microscopy, hydrodynamic diameter, and optical data for each batch to guarantee products meet specifications.
The motivation for starting nanoComposix was the frustration I had experienced as a scientist when trying to acquire high quality nanomaterials. Every time I ordered a product from a nanomaterial or chemical company I would spend many hours performing characterization only to realize that the shape, size distribution, agglomeration state, or chemical purity was not what I was expecting. I would be forced to discard the material and try another vendor. This was a time-consuming and completely unnecessary impediment to the development of technologies and products that leverage the new and exciting properties of nanomaterials.
At nanoComposix, we provide extensive characterization data with each product that we sell. Additionally, our diverse team of scientists can provide solutions to the integration problems that are a main challenge for leveraging novel nanoscale properties into commercial products. It is my hope that with improved access to highly characterized nanomaterials, researchers will be able to accelerate the page of nanomaterials research and products that harness the potential of nanotechnology.