The biodegradable polymer Poly (D,L-lactide-co-glycolic acid) (PLGA) is widely regarded as safe for use in medical applications, seeing common use in the creation of particles used in in vivo studies. The US Food and Drug Administration (FDA) and the European Medicine Agency (EMA) have approved PLGA for use in vaccines, drug delivery, and tissue engineering applications.1,2
Loading PLGA particles with either hydrophobic drugs or hydrophilic peptides and proteins is possible. This allows for the encapsulation of the active compound, protecting it from proteolytic degradation.
The nanoparticles’ surface can also be chemically modified to enable specific tropism or molecule release or to avoid the reticuloendothelial system (RES).
To successfully develop PLGA nanoparticles as a delivery system, it is necessary to precisely load and encapsulate active ingredients, ensuring that their subsequent release is controlled.
PLGA is used in various research areas, such as tissue engineering, bone defect healing, controlled release of encapsulated drugs, and vaccine development.
Several PLGA-based products are already on the market, specifically designed to manage the controlled release of encapsulated proteins or peptides.
The prostate cancer treatments Zoladex and Lupron Depot were approved in 1989. Since that time, hybrid poly (D,L-lactic acid) (PLA)/PLGA-based delivery systems have facilitated the development of extended-release formulations able to minimize drug side effects and reduce dosing frequency.
The FDA has approved 16 different PLA/PLGA-based products, 12 of which are formulated as microspheres. Due to their improved efficacy, safety, and dosing profiles, several of these products have become the most widely used medicines in their respective categories.
Formulation of PLGA nanoparticles
Several different procedures are used to prepare PLGA nanoparticles. These procedures range from traditional emulsification-solvent evaporation to more advanced nanoprecipitation methods and microfluidic devices.
Active ingredients can be encapsulated inside the nanoparticle's core, trapped among polymer chains, or adsorbed onto the nanoparticle’s surface.
Emulsification-evaporation (oil-water or water-oil-water emulsion)
Emulsification-evaporation is the most common means of preparing PLGA nanoparticles, and this technique can accommodate the encapsulation of both hydrophilic and hydrophobic drugs on the microscale or nanoscale.
In this technique, PLGA is dissolved into an organic phase (oil), which is then emulsified with a surfactant or stabilizer in an oil-immiscible phase (typically water). Hydrophobic drugs can be added to the oil phase directly, while hydrophilic drugs may be emulsified with the polymer solution before particle formation occurs.
High-intensity sonication bursts can prompt the formation of small polymer droplets. The resulting emulsion can then be added to a larger aqueous phase before stirring for several hours, permitting the solvent to evaporate.
As the solvent is removed, the polymer precipitates. Hardened nanoparticles can then be collected and washed via centrifugation before lyophilization and long-term storage.
PLGA offers an array of benefits to drug delivery, but it can be challenging to ensure the reproducible formation of nanoparticles. Variability in particle size and encapsulation efficiency can be introduced through different equipment, precursor reagent batches, and emulsification methods.
The evaporation step also necessitates heat and vacuum, potentially introducing further variability in the resultant particles. The figure below provides an overview of the double emulsion procedure (water/oil/water, w1/o/w2) used to obtain PLGA microparticles or nanoparticles.
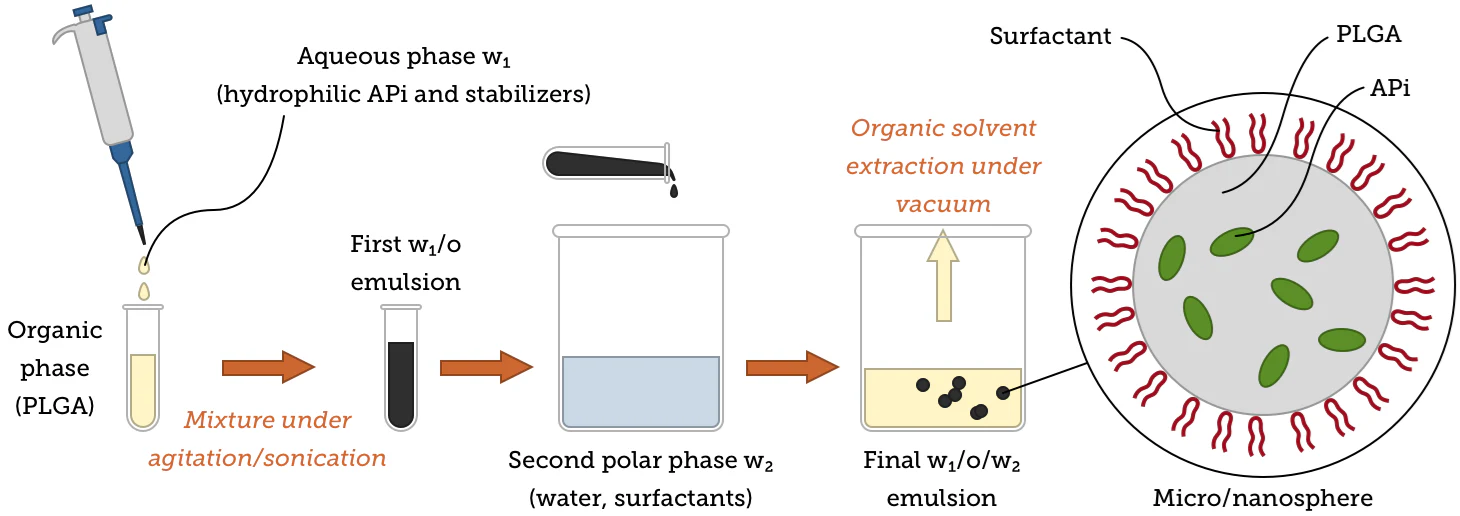
Image Credit: nanoComposix
Adjusting synthesis conditions can help facilitate the generation of microspheres or nanospheres with a uniform matrix of microcapsules/nanocapsules with core-shell structures, for example, by changing the stabilizers, solvents, and mixing procedures used. Immunoparticles employed in targeted delivery can also be acquired by attaching certain antibody molecules to particle surfaces.
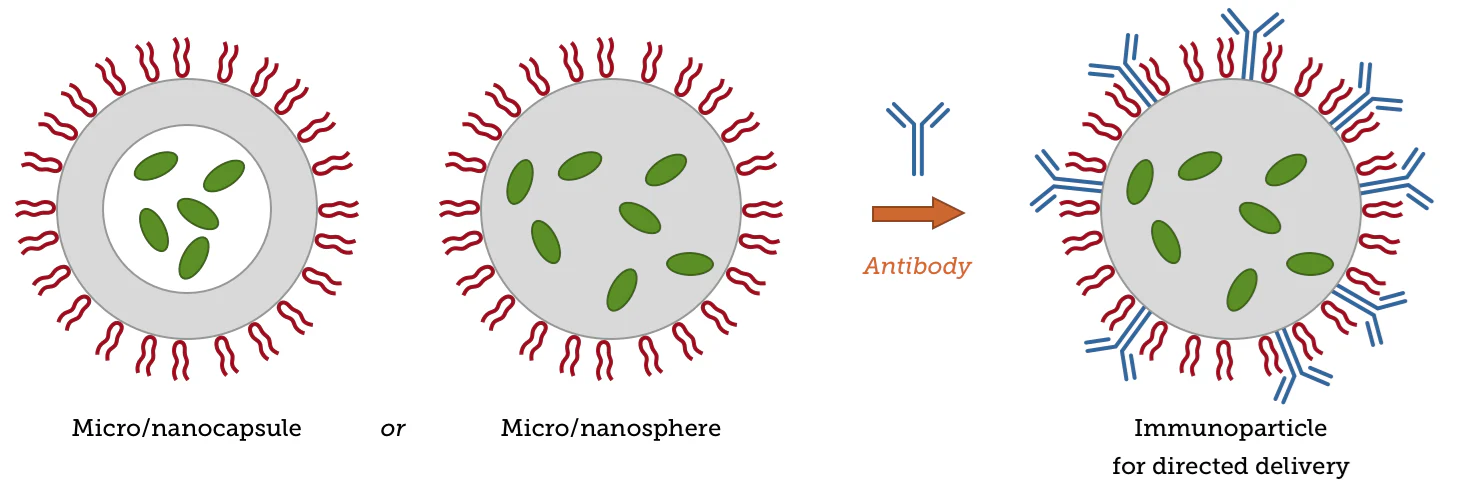
Image Credit: nanoComposix
Nanoprecipitation
Nanoprecipitation - also referred to as interfacial deposition - is another popular approach to the preparation of PLGA nanoparticles. This method sees both the polymer and the drug dissolved in an organic solvent (for example, acetone or DMSO) before being added dropwise to water.
Once the organic solvent has evaporated, centrifugation is used to collect the remaining particles as a pellet. This simple, one-step process was initially applied to hydrophobic drugs, partly due to its high reproducibility.
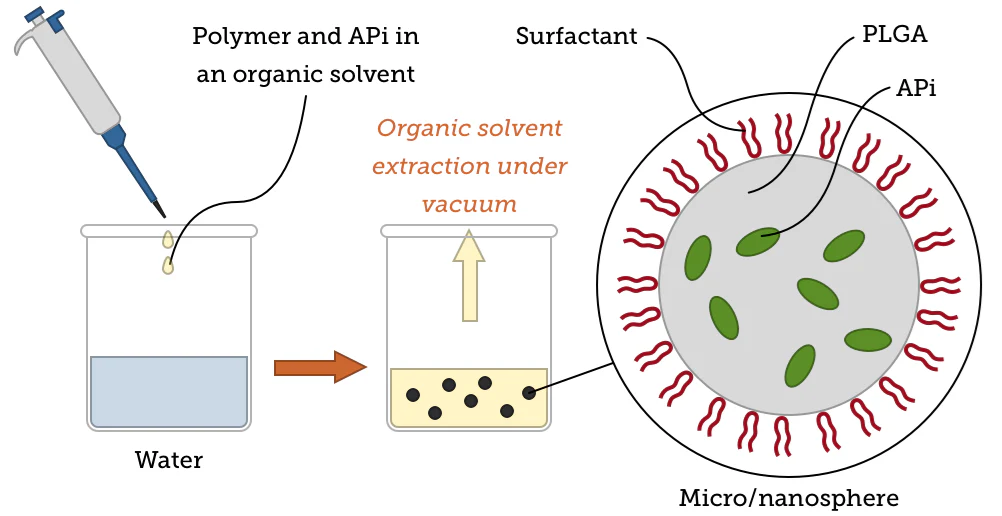
Image Credit: nanoComposix
The figure below features TEM images of PLGA nanoparticles prepared at nanoComposix. These particles were prepared using a one-step nanoprecipitation self-assembly method. Particle measurements are provided below the images.
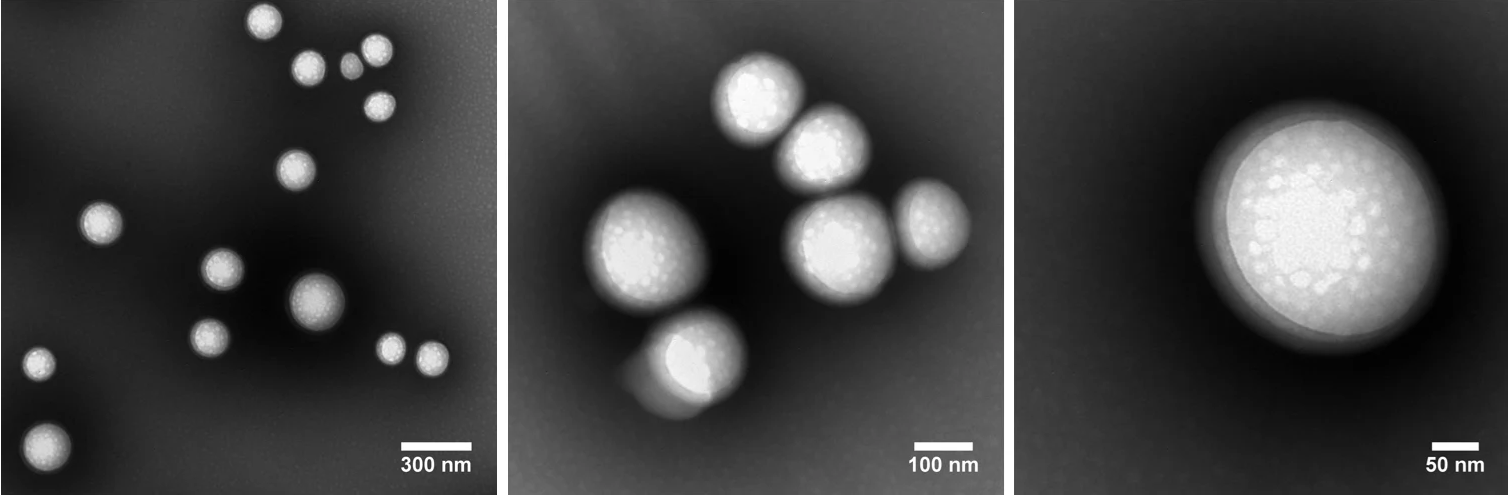
Image Credit: nanoComposix
DLS measurements: Z-Ave = 233.3 nm, Std. Dev. = 3.413, PDI = 0.062
TEM results: Ave. Diam. = 193.3 nm; Std. Dev. = 63.7; CV = 32.9%
Nanoprecipitation has a number of advantages and disadvantages as a synthetic method. Particle size distributions are extremely sensitive to mixing effectiveness during synthesis, and this method can yield particles that suffer from reportedly low drug loading; particularly when encapsulating hydrophilic components such protein and peptides.3
nanoComposix significantly improves encapsulation efficiency through a range of innovative adjustments; for example, variations in pH,4 the incorporation of salt additives or oil solutions5, or the use of a revised nanoprecipitation method.6 It should also be noted that studies demonstrate that more efficient encapsulation can be achieved via nanoprecipitation than via emulsion-based methods.7
The figure below features TEM images of PLGA nanoparticles that have been loaded with human serum albumin (HSA). These nanoparticles were prepared via a revised nanoprecipitation method at nanoComposix. Particle measurements are also provided.
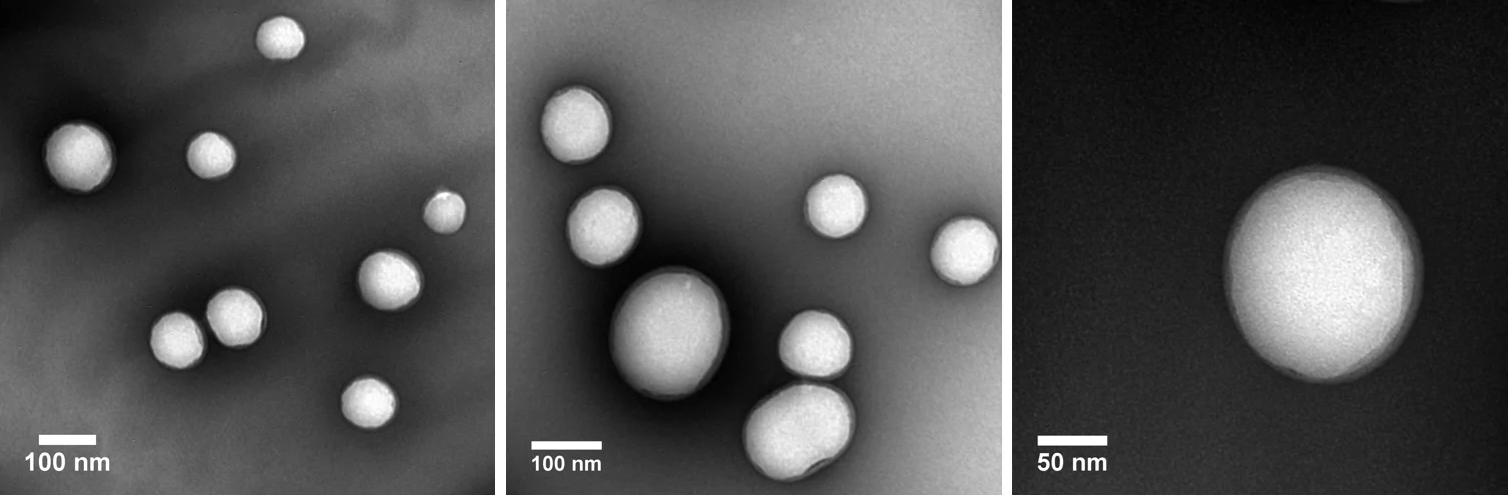
Image Credit: nanoComposix
DLS measurements: Z-Ave = 172.3 nm, Std. Dev. = 3.413, PDI = 0.076
TEM results: Ave. Diam. = 112 nm; Std. Dev. = 31.1; CV = 27.8 %
Flash nanoprecipitation
The flash nanoprecipitation approach was recently developed to generate polymeric nanoparticles via rapid micro-mixing. Its primary aim is to improve throughput compared to nanoprecipitation.
Flash nanoprecipitation uses specially designed flow geometries, such as a multi-inlet vortex mixer or a confined impinging jet mixer. Images and diagrams presented below (adapted from Reference 8) showcase a standard vortex mixer design.
The mixer (left) supplies the fluid to be mixed using four syringes, while the vortex mixer itself is comprised of four ports in a radial configuration. This instrument can process up to 100 mL of material per minute.
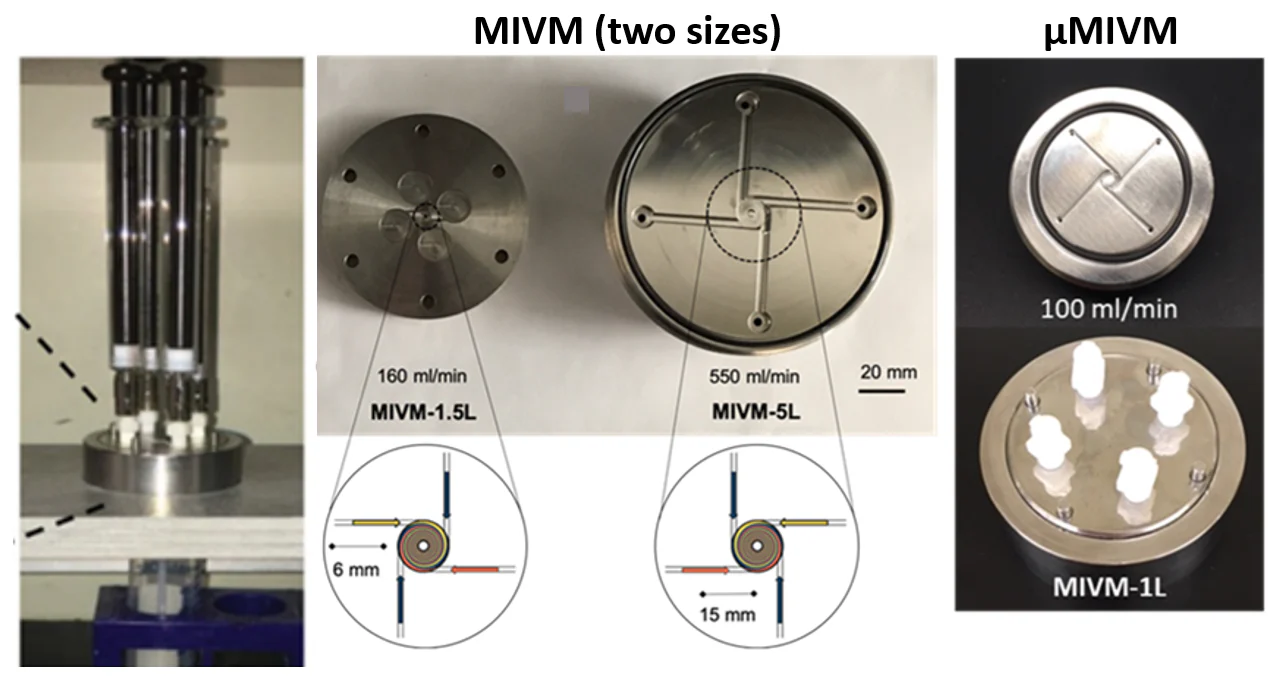
Image Credit: nanoComposix
The streams of fluid containing the different components come into contact in the mixer, and these are subjected to turbulent high shear conditions. The resulting change in solubility afforded by the solvent mixture induces rapid coprecipitation of both the cargo and the nanoparticles.
Flash nanoprecipitation represents an accessible lab-scale screening tool that is sufficiently scalable to enable nanoparticle production for translational research. It can also be used to create polymeric nanoparticles comprised of either a hydrophilic or hydrophobic core.
Under typical operating conditions, over 95% of the core material is generally encapsulated in the particle at a high mass fraction. Users wishing to process larger batch sizes should run the process for longer.
This helps avoid the mixing problems commonly linked to traditional nanoprecipitation methods, better enabling the scale-up of lead formulations via syringe pumps or flow controllers.
Microfluidics-assisted synthesis
Microfluidics-assisted synthesis has the potential to produce uniform, reproducible nanoparticles, but this method is typically limited to production on the milligram scale. Scaling up this method involves the implementation of expensive automatic parallelization processes.
This approach sees an intimate mixture of PLGA and its cargo suspended in a non-solvent state before being forced at very high pressure by a small pore above the PLGA’s glass transition temperature.
This configuration generates high shear forces, reducing the size of the PLGA particles. The subsequent addition of a coating traps the particle at a particular size while preventing particle agglomeration. Typical coatings used in this method include polyvinyl alcohol (PVA).
Image Credit: nanoComposix
Characterizing and manufacturing PLGA at nanoComposix
nanoComposix leverages an array of analytical capabilities in the characterization of PLGA. It is possible to characterize particle morphology using transmission electron microscopy (TEM) and scanning electron microscopy (SEM).
Optimal visualization can be achieved via TEM by negatively staining PLGA particles using either Nano-W from Nanoprobes or ammonium molybdate. A Zetasizer Nano-ZS from Malvern Analytical measures size distribution, polydispersity index, and charge (zeta potential), while nanoparticle tracking analysis (NTA) quantifies particle concentrations.
Fourier-transform infrared (FTIR) spectroscopy is used to confirm chemical structure, while sterility is characterized by sensitive endotoxin detection methods with a detection limit of 0.001 EU/mL in diluted samples.
Drug loading and encapsulation efficiency are determined spectrophotometrically using UV-Vis, fluorescence, or specific assays.
Mass balance measurements are employed in the determination of reaction yields, while the residual PVA associated with PLGA nanoparticles is quantified spectrophotometrically using an iodine assay developed in-house.
Release kinetics can be characterized in phosphate-buffered saline (PBS) or using a customer-specific solvent or buffer.
Process optimization to facilitate scale-up
Many of these techniques are not amenable to large-scale operation, most notably vortexing, evaporation, centrifugation, and sonication. To address this issue, nanoComposix has made its own modifications to the methods outlined in this article, allowing the company to notably improve its scalability.
The mixing process can be switched to an impinging flow mixer to better satisfy the need for homogenization. For example, an impinging flow mixer could replace single or double emulsion with nanoprecipitation.
Simple solvent evaporation can be inefficient and impractical at large scales. To address this limitation, nanoComposix works with several rotary evaporators to enable rapid solvent removal for both small-scale and large-scale synthesis.
Centrifugation for particle purification also presents scaling challenges, particularly when spinning at high speeds using ultracentrifugation. nanoComposix has created several proprietary methods specially designed for high-throughput nanoparticle processing, enabling the efficient washing and removal of any remaining solvent and unreacted precursors.
Uneven energy exposure can be problematic for bath sonication at scale. To generate the required high shear force and ensure final dispersion, the sonication step can be replaced with a Pasteur probe, high shear mixing, or micro-fluidization. If necessary, a larger-scale flow-through sonicator can also be employed.
The final nanoparticle product will undergo sterile filtration. Other sterilization methods utilize gamma radiation or E-beam. Endotoxin levels are also tested as a final stage of the company’s routine characterization procedures.
nanoComposix lyophilizes the colloidal suspensions to ensure particle stability and efficient storage. Sugars are present as the freeze-drying/lyophilization process is undertaken because these act as a cryoprotectant to avoid particle aggregation.
PLGA nanoparticle production under GMP
nanoComposix offers a range of services to enable the production of PLGA particles at scale. These services are ideally suited to the supply of material for pre-clinical and Phase I/Phase II clinical trials looking to adhere to GMP and ISO13485 guidelines.
Scale-up of synthesis can be accommodated to produce precise lot sizes for clinical trials or other customer requirements.
Particles are generally formulated under aseptic conditions to achieve sterility. Gamma radiation or electronic beam can also be applied following synthesis, provided the process will not ionize the active ingredients in the active pharmaceutical ingredient (API).
Particles are routinely tested before and after radiation exposure to ensure that the sterilization process has not compromised performance.
Particles are optimized for performance and manufacturability to a design freeze, including initiation of design control.
The company also provides comprehensive quality system documentation on particle synthesis and characterization. This documentation includes particle synthesis with GMP controls and internal auditing for quality control.
A 6-18-month timeline is typical for particle production under GMP control.
References and further reading
- Danhier, F.; Ansorena, E.; Silva, J. M.;Coco, R.; Breton, A. L.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control Release 2012, 161, 505–522. doi.org/10.1016/j.jconrel.2012.01.043.
- Allahyari, M., Mohit, E. Peptide/protein vaccine delivery system based on PLGA particles. Hum. Vaccines Immunother. 2016, 12 (3), 806-828. doi.org/10.1080/21645515.2015.1102804
- Rezvantalab S.; Drude, N. I.; Moraveji, M. K.; Güvener, N.; Koons, E. K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front Pharmacol.2018, 9, 1260. doi.org/10.3389/fphar.2018.01260.
- Govender, T.; Stolnik, S.; Garnett, M. C.; Illum, L.; Davis, S. S. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J. Control Release 1999, 57 (2), 171–185. doi.org/10.1016/S0168-3659(98)00116-3.
- Dalpiaz, A.; Vighi, E.; Pavan, B.; Leo, E. Fabrication via a nonaqueous nanoprecipitation method, characterization and in vitro biological behavior of N6‐cyclopentyladenosine‐loaded nanoparticles. J. Pharm. Sci. 2009, 35, 1375-1383. doi.org/10.1002/jps.21710
- Niu, X.; Zou, W.; Liu, C.; Zhang, N.; Fu, C. Modified nanoprecipitation method to fabricate DNA-loaded PLGA nanoparticles. Drug Dev. Ind. Pharm. 2009, 35 (11), 1375-1383. doi.org/10.3109/03639040902939221.
- Alshamsan, A. Nanoprecipitation is more efficient than emulsion solvent evaporation method to encapsulate cucurbitacin I in PLGA nanoparticles. Saudi Pharm. J. 2009, 201422, 219–222. doi.org/10.1016/j.jsps.2013.12.002.
- Markwalter, C. E.; Pagels, R. F.; Wilson, B. K.; Ristroph, K. D.; Prud’homme, R. K. Flash NanoPrecipitation for the Encapsulation of Hydrophobic and Hydrophilic Compounds in Polymeric Nanoparticles. Jove-J Vis Exp 2019, 143, e58757. doi.org/10.3791/58757.
 About nanoComposix
About nanoComposix
Since 2004, nanoComposix has provided monodisperse and unagglomerated metal and metal-oxide nanomaterials to thousands of customers. Hundreds of different variants of material, size, shape, and surface are available as stock products and we have produced over 2000 custom core/shell, biofunctionalized, fluorescent, and magnetic nanocomposites to meet client specifications. nanoComposix produced the NIST nanosilver reference material and our particles have been utilized in over 2000 peer-reviewed publications. All of our materials are supplied with certificates of analysis that include electron microscopy, hydrodynamic diameter, and optical data for each batch to guarantee products meet specifications.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.