This mRNA-lipid nanoparticle (LNP) vaccine encoded a full-length nucleocapsid (N) protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ancestral strain Wuhan-Hu1. They evaluated its immunogenicity and efficacy in mice and hamster models against all SARS-CoV-2 variants of concern (VOCs) alone and combined with the current clinically used mRNA-based vaccines based on spike (S) protein.
 Study: Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Image Credit: Orpheus FX / Shutterstock
Study: Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Image Credit: Orpheus FX / Shutterstock
Background
All COVID-19 vaccines combating SARS-CoV-2 infections target the SARS-CoV-2 S protein or its receptor binding domain (RBD) for eliciting a potent neutralizing antibody (nAb) response. Thus, the researchers hypothesized that a vaccine targeting a more conserved SARS-CoV-2 protein or multivalent vaccines would provide broader protection against newly-emerging highly mutated SARS-CoV-2 variants. The SARS-CoV-2 N protein is a highly conserved and potent immunogen shown to trigger a strong T cell response, which makes it an ideal candidate for incorporation into next-generation vaccines.
About the study
In the present study, researchers evaluated the immunogenicity of mRNA-N vaccine formulation in BALB/c mice. They created two groups, with seven mice each, and vaccinated them with phosphate-buffered saline (PBS) (mock) or 1 μg of m-RNA N vaccine intramuscularly (IM) at week zero (prime) and week 3 (booster). Following primary vaccination, the team collected serum samples for antibody analysis. After booster vaccination, they euthanized mice for further immunological analyses.
The team examined the T cell responses in splenocytes by flow cytometry. Likewise, they measured the N-specific T cell response by intracellular cytokine staining (ICS) of splenocytes. In addition, they performed an interferon-gamma (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay to evaluate the mRNA-N vaccine-induced T cell responses.
Furthermore, the researchers used an enzyme-linked immunosorbent assay (ELISA) to determine antibody titers of N-specific–binding immunoglobulin G (IgG). The team performed similar vaccine evaluations against the SARS-CoV-2 Delta VOC in Syrian hamsters.
Study findings
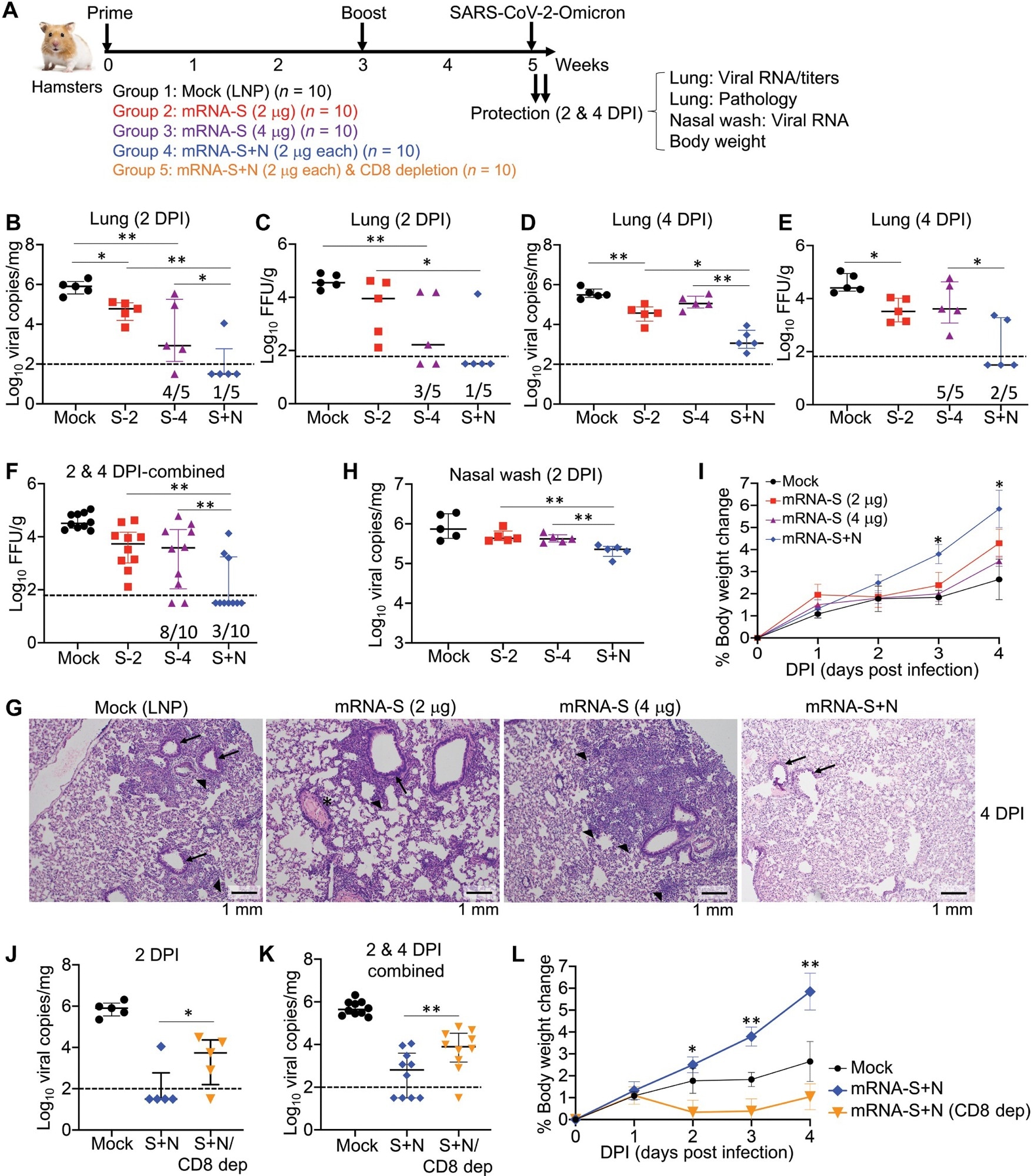
The mRNA-N was highly immunogenic but only moderately controlled SARS-CoV-2 infection. However, the combination mRNA-S+N vaccination more robustly controlled the SARS-CoV-2 Delta and Omicron VOCs in the lungs of infected mice than mRNA-S alone and provided additional protection against both variants resulting in reduced viral load in their upper respiratory tract (URT).
 Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models
Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models
The study provided considerable evidence suggesting the involvement of T cells in the mRNA-S+N vaccine-induced protection against SARS-CoV-2 variants. For instance, mRNA-N alone induced modest protection against both SARS-CoV-2 and Delta strains in the absence of neutralizing antibodies. Likewise, the results of in vivo cell depletion analysis suggested the potential involvement of a cluster of differentiation 8 (CD8+) T cells in the mRNA-S+N vaccine-induced immune protection. The authors performed an antigen-specific immune analysis and observed that the induction of N-specific immunity with enhanced S-specific immunity helped bivalent mRNA vaccine mount a more vigorous immune response.
Intriguingly, the mRNA S-based vaccine and the combination vaccine (mRNA-S+N) had similar mRNA-S doses, yet, it augmented S-specific immunity. One hypothesis is that cross-priming effects occurred between N and S antigens following vaccination by the mRNA-S+N vaccine. It is also likely that mRNA-N co-immunization induced an immune environment that favored the development of S-specific immunity. However, future studies should investigate all the events following combined mRNA-S+N vaccination, including antigen presentation and stimulation of the innate and inflammatory responses.
Conclusions
The study highlighted that since the mRNA-LNP platform has been tested and shown a favorable safety profile in multiple clinical studies in humans, this approach could be rapidly made clinically viable against yet-to-emerge SARS-CoV-2 VOCs. Previous studies have demonstrated challenges in designing COVID-19 vaccines with VOC-specific sequences. The vaccine tested in the current study had mRNA-N and mRNA-S amino acid sequences from the Wuhan-Hu-1. Yet, it elicited robust protection against both Delta and Omicron VOCs, which was exemplary. Further testing of the combination vaccine approach in non-human primates (NHPs) would provide more opportunities to evaluate its safety and efficacy.
In hamsters challenged by SARS-CoV-2 VOCs, the combined mRNA-S+N vaccine induced robust viral control in the lungs. However, its additive antiviral effect appeared to diminish in URT. Therefore, future studies should investigate heterologous vaccination approaches involving different vaccine platforms and immunization routes. For instance, vaccination strategies using IM, intranasal and oral delivery routes to improve protection against VOCs in the URT.
Journal reference:
- Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models, Renee L. Hajnik, Jessica A. Plante, Yuejin Liang, Mohamad-Gabriel Alameh, Jinyi Tang, Srinivasa Reddy Bonam, Chaojie Zhong, Awadalkareem Adam, Dionna Scharton, Grace H. Rafael, Yang Liu, Nicholas C. Hazell, Jiaren Sun, Lynn Soong, Pei-Yong Shi, Tian Wang, David H. Walker, Jie Sun, Drew Weissman, Scott C. Weaver, Kenneth S. Plante, Haitao Hu, Science Translational Medicine 2022, DOI: 10.1126/scitranslmed.abq1945, https://www.science.org/doi/10.1126/scitranslmed.abq1945