In Ontario, Paxlovid has been widely available since April 2022. The therapeutic has received universal funding for all COVID-19 patients residing in community-based settings with authorization for use only in SARS-CoV-2-infected individuals with advanced age, comorbidities, and under-vaccinated.
 Study: Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada. Image Credit: NIAID
Study: Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
To inform future policies and guidelines, it is critical to collect observational data evaluating the real-world use of this medication.
Using a population-based observational cohort study, researchers evaluated Paxlovid's effectiveness in reducing hospitalizations and deaths from COVID-19, while Omicron and its subvariants are predominant.
The study comprised Canadian residents aged between 18 years and 110 years who were diagnosed as SARS-CoV-2-positive by polymerase chain reaction (PCR) between April 4 and August 31, 2022. The team compared Paxlovid-treated SARS-CoV-2-positive individuals to untreated COVID-19 patients and assessed the prime outcome of COVID-19-associated hospitalizations or deaths and the secondary study outcome pertaining to deaths within one to 30 days.
The team excluded individuals who did not reside in Ontario, whose identifier data such as the birth date was invalid, or if the death date was before the SARS-CoV-2 testing. In addition, hospital-admitted patients or those with nosocomial infections were excluded from the analysis. Weighted logistic regression modeling was used for the analysis. The weighted odds ratio (wOR) values were calculated using IPTW (inverse probability of treatment weighting) for propensity scores to account for confounding variables.
Paxlovid prescription data, SARS-CoV-2 testing data, and vaccination data were obtained from the ODB (Ontario drug benefit), C19INTGR, and COVAXON databases, respectively. Comorbidity data were obtained from the CIHI (Canadian institute for health information) databases, OHIP (Ontario health insurance plan) database, and other validated disease-specific ICES (institute for clinical evaluative sciences) cohorts. Data analysis was performed at ICES.
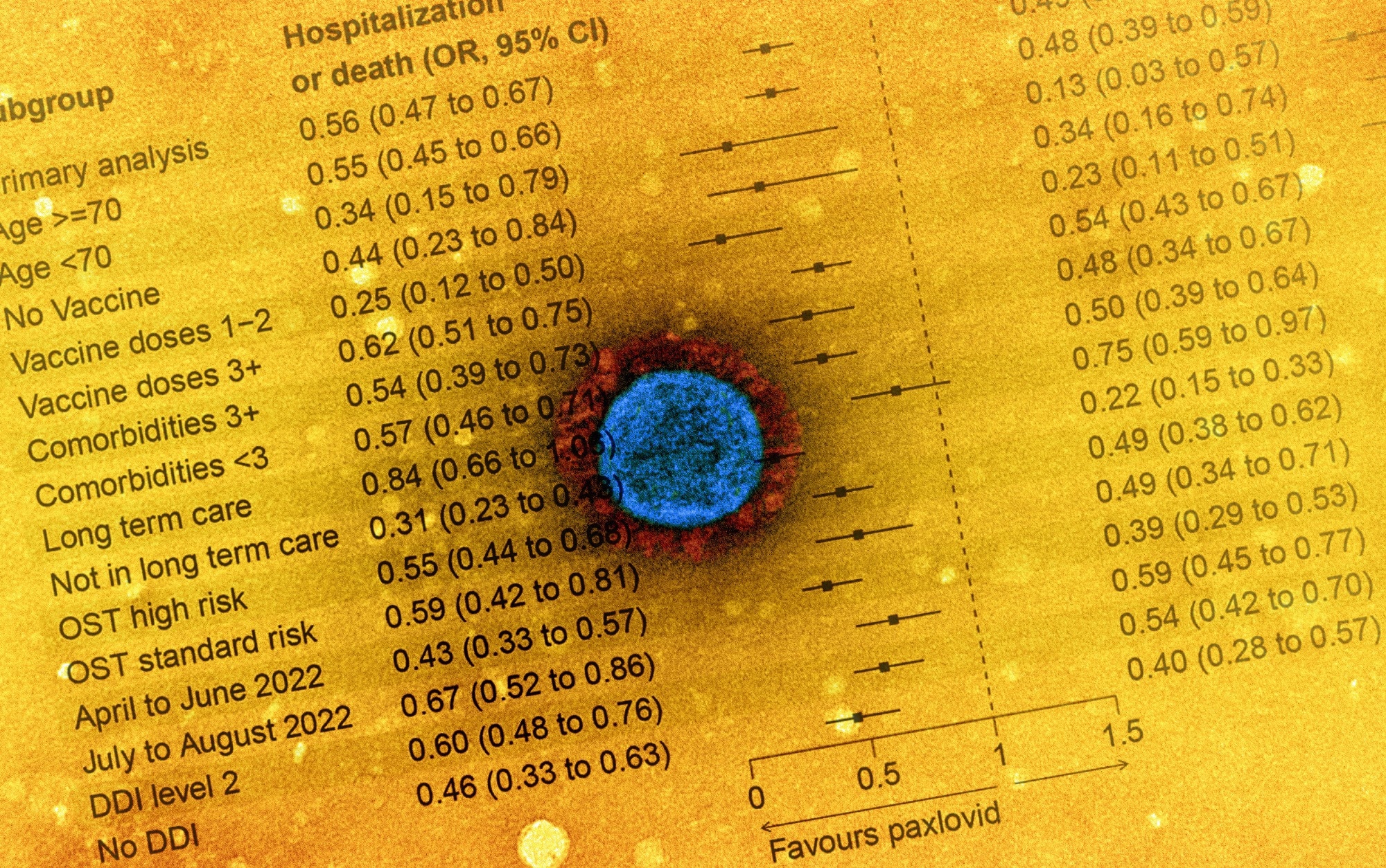
The secondary outcomes were ascertained using the CCM (case and contact management) database. In addition, subgroup analyses were performed, including age stratifications (below or above 70 years), the status of vaccination (no, one-dose, two-dose, or three or more doses received), probable drug-drug interactions (DDIs) for individuals aged >70, comorbidities, OST high risk vs. standard risk, long-term care individuals and duration (between April and June 2022 and between July and August 2022).
Results
In total, 177,545 SARS-CoV-2-positive individuals were considered for the final analysis, of which 8,876 (5%) and 168,669 (95%) were exposed and unexposed, respectively. Among Paxlovid-treated individuals, 5.0% had received COVID-19 vaccinations, the average age was 74 years, 67% of individuals aged ≥70 years had probably significant DDIs, and 32% of the treated individuals lived in long-term care settings.
Hospitalizations or deaths in the 30 days were lower among Paxlovid-treated patients compared to the unexposed patients [two percent versus four percent, equating to 44% RR (relative reduction), wOR 0.6]. In addition, the relative chances of mortality significantly lowered among treated individuals (two percent versus three percent, 51% RR, wOR 0.5).
The NNT (number needed to treat) value for preventing a single hospital admission or death from severe SARS-CoV-2 infections was 62. Considerable variability was observed in risk reductions in absolute terms by strata, with NNT values ranging between 28 for non-vaccinated individuals and 180 for individuals aged below 70 years.
The findings were comparable for all ages, DDIs, comorbidities, and the status of COVID-19 vaccination. However, a potential reduction was observed in Paxlovid treatment efficacy by time with obtained wOR values of 0.4 and 0.7 for hospitalizations or deaths from April to June 2022 and from July to August 2022, respectively, with a similar trend observed for only deaths.
The team observed a probable reduction in Paxlovid treatment efficacy in the latter 2.0 months of the study (characterized by Omicron BA.5 dominance) which could have been observed by chance, due to altering COVID-19 severity with time, or due to antiviral resistance.
Overall, the study findings showed that Paxlovid treatment significantly lowered the risks of COVID-19-associated hospitalizations and deaths, underpinning the ongoing usage of the anti-SARS-CoV-2 therapeutic for treating patients with mild SARS-CoV-2 infections at risk for severe COVID-19. The most significant risk reductions in relative and absolute terms were noted among non-vaccinated individuals aged ≥70.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Real-world effectiveness of nirmatrelvir/ritonavir use for COVID-19: A population-based cohort study in Ontario, Canada. Schwartz KL MD MSc et al. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.11.03.22281881, https://www.medrxiv.org/content/10.1101/2022.11.03.22281881v1
- Peer reviewed and published scientific report.
Schwartz, Kevin L., Jun Wang, Mina Tadrous, Bradley J. Langford, Nick Daneman, Valerie Leung, Tara Gomes, Lindsay Friedman, Peter Daley, and Kevin A. Brown. 2023. “Population-Based Evaluation of the Effectiveness of Nirmatrelvir–Ritonavir for Reducing Hospital Admissions and Mortality from COVID-19.” CMAJ 195 (6): E220–26. https://doi.org/10.1503/cmaj.221608. https://www.cmaj.ca/content/195/6/E220.