Background
Concerted scientific efforts led to the rapid development of coronavirus disease 2019 (COVID-19) vaccines using several platforms, the most successful being messenger ribonucleic acid (mRNA)-based vaccines. Subsequently, ChAdOx1 nCoV-19, an adenovirus-based, and BNT162b2, an mRNA vaccine, received approval for use in the U.K. on December 8, 2020, and December 31, 2020, respectively. Real-world data showed that these vaccines were highly effective against symptomatic COVID-19, severe illness, and hospitalization.
Yet, some rare adverse events, such as thrombosis, were reported following vaccination during immunization programs. As of May 26, 2021, the U.K. reported thromboembolic events with thrombocytopenia following primary vaccination using ChAdOx1 nCoV-19 and BNT162b2. Importantly, fewer safety concerns for the BNT162b2 have been reported, with instances of immune thrombocytopenia remaining rare among BNT162b2 recipients.
About the study
In the present study, researchers assessed six cohorts of individuals from the U.K. general population to study the effect of COVID-19 vaccination. Four vaccinated cohorts included individuals vaccinated with ChAdOx1 or BNT162b2 vaccines. These individuals received either their first or second dose between December 8, 2020, and May 2, 2021, and were followed up with until 28 days from the index date.
The fifth cohort comprised unvaccinated individuals who contracted COVID-19 between September 1, 2020, and May 2, 2021, as confirmed by a reverse transcription-polymerase chain reaction (RT-PCR) test. All study participants in this cohort were followed up with up to 90 days following their initial diagnosis.
The sixth cohort, which was otherwise known as the general population background cohort, included people from Clinical Practice Research Datalink (CPRD) AURUM as of January 1, 2017. The team followed up with this cohort up to December 31, 2019.
Study participants older than 20 years were included in the primary analysis and provided their prior medical history of at least one year.
Diagnostic codes were used to identify five venous thromboembolic events including cerebral venous sinus thrombosis (CVST), pulmonary embolism (PE), splanchnic vein thrombosis (SVT), deep vein thrombosis (DVT), and the composite event venous thromboembolism (VTE), which encompassed DVT and PE. Two arterial thromboembolic events (ATE) including myocardial infarction and ischemic stroke were also identified.
All cases meeting the Brighton collaboration definition were considered cases of thrombocytopenia. Moreover, thrombocytopenia patients had a platelet count between 10,000 and 150,000 platelets per microliter. When thrombocytopenia occurred within 10 days before or after thrombosis, these cases were considered thrombosis with thrombocytopenia syndromes (TTS).
The number of events and observed and crude incidence rates (IRs) per 100,000 person-years were reported with 95% confidence intervals (CIs), in addition to a measure of absolute risk. Further, standardized incidence ratios (SIRs) were computed with 95% CIs by comparing observed and expected rates. All analyses were stratified by 10-year age bands and gender and for the vaccinated group by calendar month.
Study findings
The study included 3,768,517 and 1,832,841 people vaccinated with ChAdOx1, and BNT162b2, respectively, in addition to 401,691 and 9,414,403 SARS-CoV-2-infected and the general public, respectively. The cohorts comprising vaccinated populations had more women, were older, and had a higher prevalence of comorbidities, whereas those infected with SARS-CoV-2 were younger than the general population.
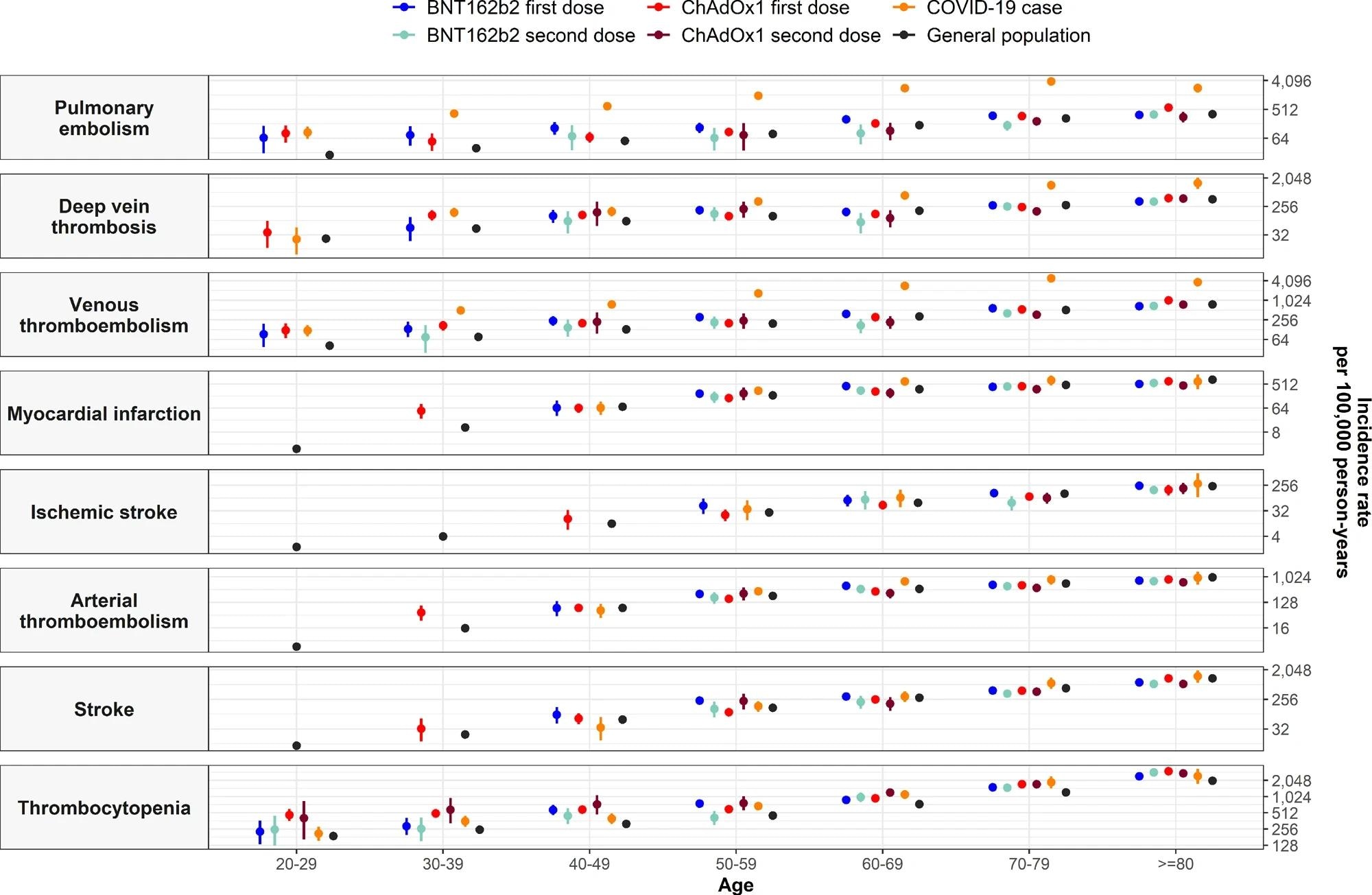 Incidence rate ratios (IRRs) for thromboembolic events and thrombocytopenia by age.
Incidence rate ratios (IRRs) for thromboembolic events and thrombocytopenia by age.
An increased risk of VTE was reported after the first dose of ChAdOx1 and BNT162b2 and with patient age across all study cohorts. Following first doses with both ChAdOx1 and BNT162b2, expected vs. observed VTE events were 771 and 866 and 533 and 595, respectively, with SIRs of 1.12. Conversely, expected vs. observed rates did not increase after the second dose of either vaccine.
Among first-dose recipients of both vaccines, IRRs increased among younger age groups, with IRRs reaching one for all age groups in the SARS-CoV-2 RT-PCR-positive cohort. This cohort had observed vs. expected VTE of 1090 and 150, with a SIR of 7.27.
PE, rather than DVT events, drove higher than expected VTE rates after the first dose of ChAdOx1 and BNT162b2. In the SARS-CoV-2 RT-PCR cohort, higher rates were due to DVT as compared to PE events. Furthermore, the SIR for PE after a SARS-CoV-2 RT-PCR positive test was 12.77.
Likewise, observed vs. expected rates for CVST were higher after the first dose of ChAdOx1 and a SARS-CoV-2-positive RT-PCR test at 16 vs. four and five vs. one, with respective SIRs of 4.14 and 3.74. SVT observed vs. expected rates for positive SARS-CoV-2 RT-PCR test were eight and three.
Rates of ATE after vaccination were not higher than expected but increased after a positive SARS-CoV-2 RT-PCR test. Notably, the expected ATE was 134 but increased to 186 after a positive SARS-CoV-2 RT-PCR test. This increased risk was more pronounced among 50- to 79-year-olds and was primarily driven by the risk of myocardial infarction.
Across all study cohorts, thrombocytopenia was more common than expected, with SIRs for BNT162b2 first dose and ChAdOx1 second dose of 1.27 and 1.47, respectively. The researchers noted more VTE events with concurrent thrombocytopenia than with ATE.
The observed vs. expected rates for VTE were higher after the first dose of ChAdOx1 at 16 vs. 12, with a SIR CI of 1.38. Rates of VTE with thrombocytopenia were relatively higher than expected after a positive SARS-CoV-2 RT-PCR test.
Conclusions
Previous studies have reported thrombocytopenia post-vaccination against influenza, measles, mumps, and rubella. Case series have also suggested that COVID-19 vaccines, especially the adenoviral-based vaccines, accentuate the risk of thrombosis and thrombocytopenia, alone or combined.
In the current study, researchers observed similar rates of arterial events among COVID-19-vaccinated people. However, further characterization enabled the researchers to show that such people were generally older and often had a prior history of related conditions or medications.
Nevertheless, the benefits of COVID-19 vaccines outweigh the risks, as the incidence of all adverse events among vaccinated people was rare.
Journal reference:
- Burn, E., Li, X., Delmestri, A. et al. (2022). Thrombosis and thrombocytopenia after vaccination against and infection with SARS-CoV-2 in the United Kingdom. Nature Communications 13(7167). doi:10.1038/s41467-022-34668-w