After two years of Coronavirus disease 2019 (COVID-19) being declared a pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to cause a significant number of infections despite many individuals being immunized or naturally infected with the virus. The presence of neutralizing antibodies is generally correlated with being immune protected from SARS-CoV-2 infection. These antibodies bind the receptor-binding domain (RBD) of the spike protein and prevent the entry of the virus inside human cells. Moreover, T cells have also been reported to play an important role in SARS-CoV-2 infection.
Several studies indicated that COVID-19 vaccination and SARS-CoV-2 infection form neutralizing anti-spike antibodies and robust T-cell responses against several viral epitopes. Such responses were detectable up to one-year post-immunization. However, a significant decrease was observed within the first few months. This can explain why several immunized individuals get re-infected with the virus.
Along with the waning of immunity, the emergence of SARS-CoV-2 variants of concern (VOC), including Omicron (B.1.1.529 lineage) and Delta (B.1.617.2 lineage), can cause such re-infections. These VOCs comprise mutations in the spike protein, preventing neutralizing antibodies from binding them. Similar observations were found for Omicron subvariants BA.4 and BA.5, where neutralizing antibodies formed after Omicron BA.1 or BA.2 infections cannot bind the newer subvariants.
A new study in the journal Vaccines aimed to analyze the long-term kinetics of SARS-CoV-2 specific T cell and humoral responses following primary and booster vaccinations in previously infected individuals. The study also compared such responses with infection-naïve vaccinated individuals and determined whether cross-reactive T cell responses are induced against the spike protein of Delta and Omicron BA.1 and BA.2 VOC due to vaccination and previous infection.
About the study
The study involved previously infected healthcare workers (HCWs) who tested SARS-CoV-2 positive between March 2020 and March 2021, recently infected HCWs who tested positive between December 2021 and May 2022, and infection-naïve HCWs who never tested positive. The SARS-CoV-2 antibody and T cell responses were measured in June 2020, June 2021, November 2021 (t0), December 2021 (t1), March 2022 (t2), and June 2022 (t3) for previously infected HCWs. Measurements for infection-naïve and recently infected HCWs occurred in March 2022 (t2) and June 2022 (t3).
Blood samples were collected from HCWs, followed by the isolation of peripheral blood mononuclear cells (PBMC). Then, assessment of T cell responses against the SARS-CoV-2 spike subunit 1 (S1) and nucleocapsid protein (N) took place by ELIspot assay followed by a SARS-CoV-2 variant IFN-γ ELISpot assay. After that, ELIspot image processing was carried out along with the quantification of spots. Next, quantitative ELISA was used to determine the serum anti-SARS-CoV-2-RBD IgG concentrations, followed by a surrogate virus neutralization assay. Finally, a chemiluminescent microparticle immunoassay (CMIA) was used to determine SARS-CoV-2 Anti-N IgG concentrations.
Study findings
The results indicated that the S1-specific T cell responses were lower at five months post-vaccination compared to 2 weeks post-vaccination, while the N-specific T cell responses were comparable between the two time points. Also, lower anti-RBD IgG serum concentrations and neutralizing activities were observed five months post-vaccination. Conversely, an increase in S1-specific T cells, along with an increase in anti-RBD IgG concentrations as well as neutralizing activity, was observed post-booster vaccination. Moreover, in previously infected individuals, strong associations were observed between serum anti-RBD IgG concentrations and neutralizing activity of serum antibodies at t1 and t0.
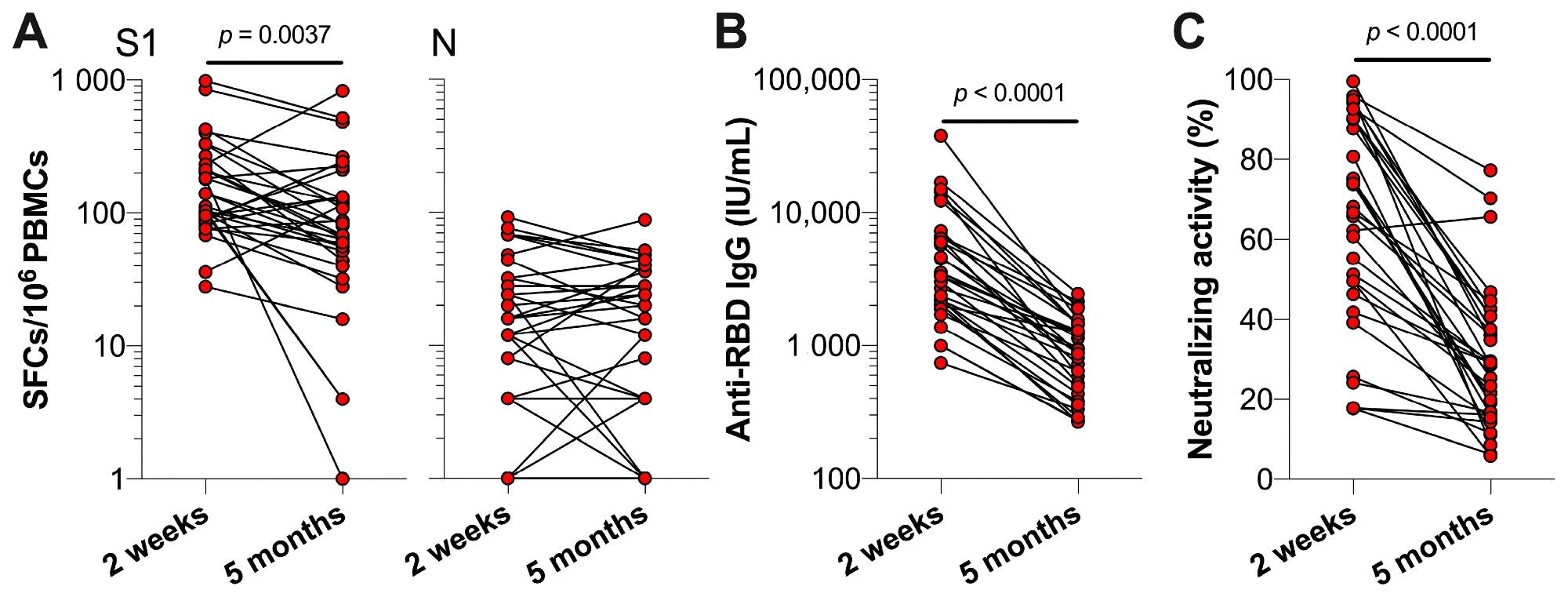 SARS-CoV-2-specific T cell and antibody responses of previously infected HCWs two weeks and five months post-primary vaccinations. Previously infected HCWs (n = 32) are represented by individual data points. (A) T cell responses against SARS-CoV-2 S1 and N, (B) serum anti-RBD IgG concentrations, and (C) the neutralizing activity of serum antibodies against SARS-CoV-2 at two weeks and five months (i.e., t0) after primary vaccination series. Statistical significance was assessed with a Wilcoxon test.
SARS-CoV-2-specific T cell and antibody responses of previously infected HCWs two weeks and five months post-primary vaccinations. Previously infected HCWs (n = 32) are represented by individual data points. (A) T cell responses against SARS-CoV-2 S1 and N, (B) serum anti-RBD IgG concentrations, and (C) the neutralizing activity of serum antibodies against SARS-CoV-2 at two weeks and five months (i.e., t0) after primary vaccination series. Statistical significance was assessed with a Wilcoxon test.
A decrease in S1-specific T cells, anti-RBD IgG concentrations, and neutralizing activity were observed four months post-booster vaccination. However, the anti-RBD IgG concentrations and S1-specific T-cell numbers were observed to be similar after three subsequent months. Additionally, the anti-RBD IgG concentrations at 4 and 7 months post-booster vaccination were relatively higher compared to 5 months post-primary vaccination.
Higher S1-specific T cell responses, anti-RBD IgG concentrations, and neutralizing activities were observed in recently and previously infected HCWs compared to infection-naïve HCWs at t2. Higher S1-specific T-cell responses were observed in previously infected HCWs compared to infection-naïve at t3, while no differences were observed between recently infected and infection-naïve HCWs. Higher N-specific T-cell responses were observed in recently and previously infected HCWs compared to infection-naïve HCWs at both t2 and t3. Moreover, higher anti-RBD IgG concentrations were also observed in recently and previously infected HCWs compared to infection-naïve HCWs at t3. Strong associations were observed between serum anti-RBD IgG concentrations and neutralizing activity of serum antibodies at t2 for all three groups.
Comparable T-cell responses were observed for the SARS-CoV-2 wild-type (WT) spike and the mutation peptide pools for all the variants. A higher Omicron BA.1 spike-specific T cell response was observed in the recently and previously infected HCWs compared to infection-naïve HCWs. Omicron BA.2 spike-specific T-cell responses were also higher for previously infected HCWs compared to infection-naïve HCWs. However, no difference was observed for Delta spike-specific T-cell responses among all the HCW groups.
Therefore, the current study demonstrates that SARS-CoV-2-specific IgG and T cell responses that wane after primary vaccination again increase after booster vaccination. Moreover, previous as well as recent infections led to higher immune responses. Vaccine-induced T-cell responses were observed to be cross-reactive against Omicron subvariants, BA.1 and BA.2. Further research should highlight whether vaccine-induced T-cell responses are also cross-reactive against the Omicron BA.5 and other emerging variants.
Limitations
The study has certain limitations. First, the sample size of each group was small. Second, the ELISpot assay is unable to characterize reactive T cells. Third, T cell reactivity was not assessed against the recent Omicron BA.5 subvariant.