NPs sized 20 – 100 nm efficiently drain into lymph nodes through the lymphatic system, where resident APCs take up NPs without needing specific cell-targeting ligands. Furthermore, recent studies demonstrated that conjugating TLR agonists to polymer NPs significantly boosts antibody synthesis and cytotoxic T-cell induction.
 Study: Nanoparticle-Conjugated TLR9 Agonists Improve the Potency, Durability, and Breadth of COVID-19 Vaccines. Image Credit: HaHanna / Shutterstock
Study: Nanoparticle-Conjugated TLR9 Agonists Improve the Potency, Durability, and Breadth of COVID-19 Vaccines. Image Credit: HaHanna / Shutterstock

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The study and findings
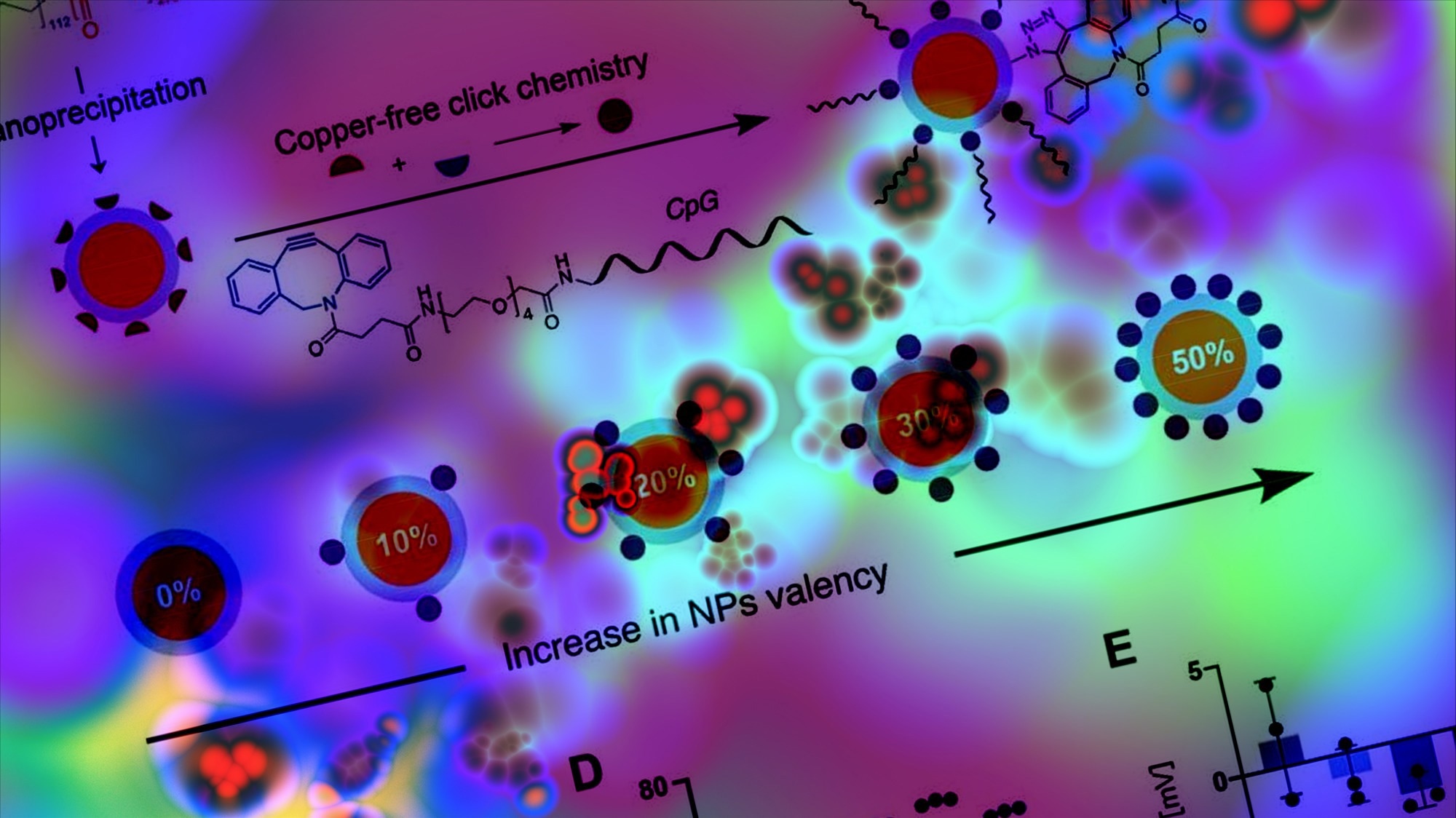
In the present study, researchers optimized CpG delivery by designing an NP-conjugated adjuvant construct to improve potency. CpG was conjugated to polyethylene glycol-b-poly lactic acid (PEG-b-PLA) with azide terminated (N3-PEG-b-PLA) NPs sized 50 nm. Next, NPs with increasing adjuvant valency were synthesized, and the resultant CpG NPs were purified.
Conjugation caused no changes in the properties of NPs, and the functionalization of CpG was confirmed. The conjugated NPs had hydrodynamic diameters (56 nm – 62 nm) within the range for improved trafficking to lymph nodes. Next, the team tested the immunogenicity and biological activity of CpG in RAW-Blue macrophage cells in vitro.
Cells were incubated with soluble CpG or CpG NPs. In addition, the assay was used to examine the effect of CpG valency on NPs and the potency of immune cell activation. Cells were incubated with soluble CpG, PEG-b-PLA NPs (plain), or CpG-conjugated NPs with variable valency. 30% CpG NP had the lowest half-maximal effective concentration (EC50), 66% lower than 10% CpG NP. The highest EC50 was obtained with 50% CpG NP, suggesting low TLR9 activation.
Therefore, 30% CpG NPs were selected for further investigation (henceforth denoted CpG NPs). Previously, the authors developed injectable polymer-NP (PNP) hydrogels that could encapsulate vaccine antigens and adjuvants and cause sustained delivery over prolonged periods. The team speculated that PNP hydrogels could further augment vaccine response.
As such, PNP hydrogels were synthesized by mixing aqueous solutions of biodegradable PEG-b-PLA NPs and hydroxypropyl methylcellulose (HPMC-C12) derivatives. Next, they analyzed PNP hydrogel formulations of 10wt% NPs (mixture of CpG NPs and unconjugated NPs) and 2wt% HPMC-C12, denoted as PNP-2-10. CpG NPs caused no significant changes in the mechanical properties of the PNP hydrogel.
These hydrogels (PNP-2-10) were synthesized and loaded with SARS-CoV-2 spike protein. The diffusivity of the spike and CpG NPs in the hydrogel were similar to the self-diffusivity of the PNP matrix, indicating that the two cargoes (CpG NP and spike) were immobilized within the hydrogel. The immunogenicity of SARS-CoV-2 spike with soluble CpG, CpG NP adjuvants, or CpG NP hydrogels was investigated.
C57BL/6 mice were primed at week 0 with vaccines comprising 10 μg spike antigen adjuvanted with soluble CpG (20 μg) or CpG NPs (containing 20 μg CpG) and boosted three weeks later with a homologous dose. In addition, animals were primed with hydrogel formulation comprising a 20 μg spike antigen with CpG NP (40 μg); unlike others, they were not boosted as the total dose was the same as a two-dose regimen.
Inflammatory cytokines were not detected immediately after immunization across all treatment groups, indicating that the formulations were well tolerated. Spike-specific immunoglobulin G (IgG) endpoint titers one-week post-immunization were lower than the detection limit for all treatment groups except the CpG NP hydrogel group. This implied faster seroconversion with hydrogel immunization.
Post-boost, the CpG NP group induced endpoint titers nearly two-fold higher than those in the soluble CpG group. The single-dose CpG NP hydrogel group had titers comparable to the two-dose CpG NP group. Moreover, the two-dose CpG NP and single-dose CpG NP hydrogel vaccines elicited higher endpoint titers against SARS-CoV-2 Beta, Delta, and Omicron variants than prime-boost soluble CpG vaccines.
Finally, the researchers evaluated the neutralizing activity of sera from each treatment group using HeLa cells and SARS-CoV-2 spike-pseudotyped lentiviral particles. At week 3, before boosting, sera from mice immunized with soluble vaccines had at least 50% infectivity, while sera from the CpG NP hydrogel vaccine group protected cells from infection.
At week 5, two weeks post-boost, sera from soluble vaccine recipients had less than 50% viral infectivity. In contrast, sera from the CpG NP hydrogel vaccine group had again protected cells from infection. The most potent neutralizing activity was observed with CpG NP vaccine sera, followed by the CpG NP hydrogel vaccine sera.
Conclusions
To summarize, the research team developed an NP-conjugated adjuvant platform whereby CpG, a TLR9 agonist, was displayed on the PEG-b-PLA NP surface. 30% CpG NPs showed the highest potency in vitro, which were used as adjuvants for vaccine candidates. The CpG NP-adjuvanted vaccines induced greater humoral responses than soluble CpG-adjuvanted vaccines.
Furthermore, the single-dose CpG NP hydrogel vaccine elicited humoral responses comparable to the two-dose regimen of the CpG NP vaccine. These findings are promising and could help lower vaccination costs and improve compliance to achieve high vaccination rates.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.