The primary pathogenic feature of PAS is the failure of the placenta to separate spontaneously from the uterus post-delivery. As such, PAS may result in catastrophic outcomes that may even cause death. Antenatal diagnosis of PAS could allow for early multidisciplinary care, reducing PAS-associated maternal morbidity by up to 80%. Nevertheless, a significant number of PAS cases are undiagnosed before delivery.
The risk for PAS is currently examined by historical/clinical risk factors and radiologic evaluation. A readily available biomarker for PAS would offer unprecedented clinical utility, particularly in rural and low-resource settings. CMPs are micro- to nano-sized extracellular lipid bilayer vesicles that mediate cell signaling and communication. CMPs have been promising in obstetrics research, especially preterm births and preeclampsia.
 Study: Circulating microparticle proteins predict pregnancies complicated by placenta accreta spectrum. Image Credit: Blue Planet Studio / Shutterstock
Study: Circulating microparticle proteins predict pregnancies complicated by placenta accreta spectrum. Image Credit: Blue Planet Studio / Shutterstock
The study and findings
In the present study, researchers in the United States investigated whether CMPs can predict pregnancies complicated by PAS. Plasma specimens obtained at a median gestation of 26 and 35 weeks were collected between 2007 and 2020 from the LIFECODES pregnancy biobank at Brigham and Women’s Hospital, Boston. Eligible subjects were patients aged 18 or older receiving prenatal care and planning on delivery at the specified hospital.
Pregnancy was confirmed by ultrasound at ≤ 12 gestation weeks. Cases were individuals with clinical or histological grade 1 – 3 PAS. Prospective cases were identified in the electronic medical record (EMR) using specific search terms between 2007 and 2020. Controls were subjects without PAS diagnosis and randomly matched 2:1 by gestational age and the number of fetuses.
Subjects were excluded if they had cancer/documented fetal chromosomal abnormality or used immunomodulators. Thirty-five cases and 70 controls were included; 27 cases had grade 1, seven had grade 2, and one had grade 3 PAS. Clinical criteria identified nearly 66% of PAS cases. CMPs were isolated using size-exclusion chromatography and identified by liquid chromatography with tandem mass spectrometry.
The team implemented a two-step iterative workflow to identify CMP proteins that would serve as markers for PAS risk in the second and third trimesters using regularized regression (L1) and logistic regression for cross-validation. The same workflow was repeated with randomly permuted samples to simulate random chance.
The average of all area under the curves (AUCs) significantly differed between observed and permuted panels in the second-trimester samples. The top-performing CMP panel differentiated PAS from controls with an average AUC of 0.83. This included the following CMP proteins: cartilage acidic protein 1 (CRAC1), hemoglobin subunit gamma 2 (HBG2), sulfhydryl oxidase 1 (QSOX1), and. histone 4 (H4), and isthmin-2 (ISM2).
Likewise, the mean of all AUCs for third-trimester samples significantly differed between observed and permuted panels. The four top-performing proteins - ISM2, immunoglobulin (Ig) lambda variables 10 – 54, Ig-like domain-containing protein, and ubiquitin carboxyl-terminal hydrolase, distinguished PAS from controls with an average AUC of 0.78.
The team further applied ingenuity pathway analysis (IPA) to ascertain the biological function of identified proteins differentially expressed by PAS status. The analysis revealed a significant overrepresentation of upstream regulators, canonical pathways, and cellular and molecular functions.
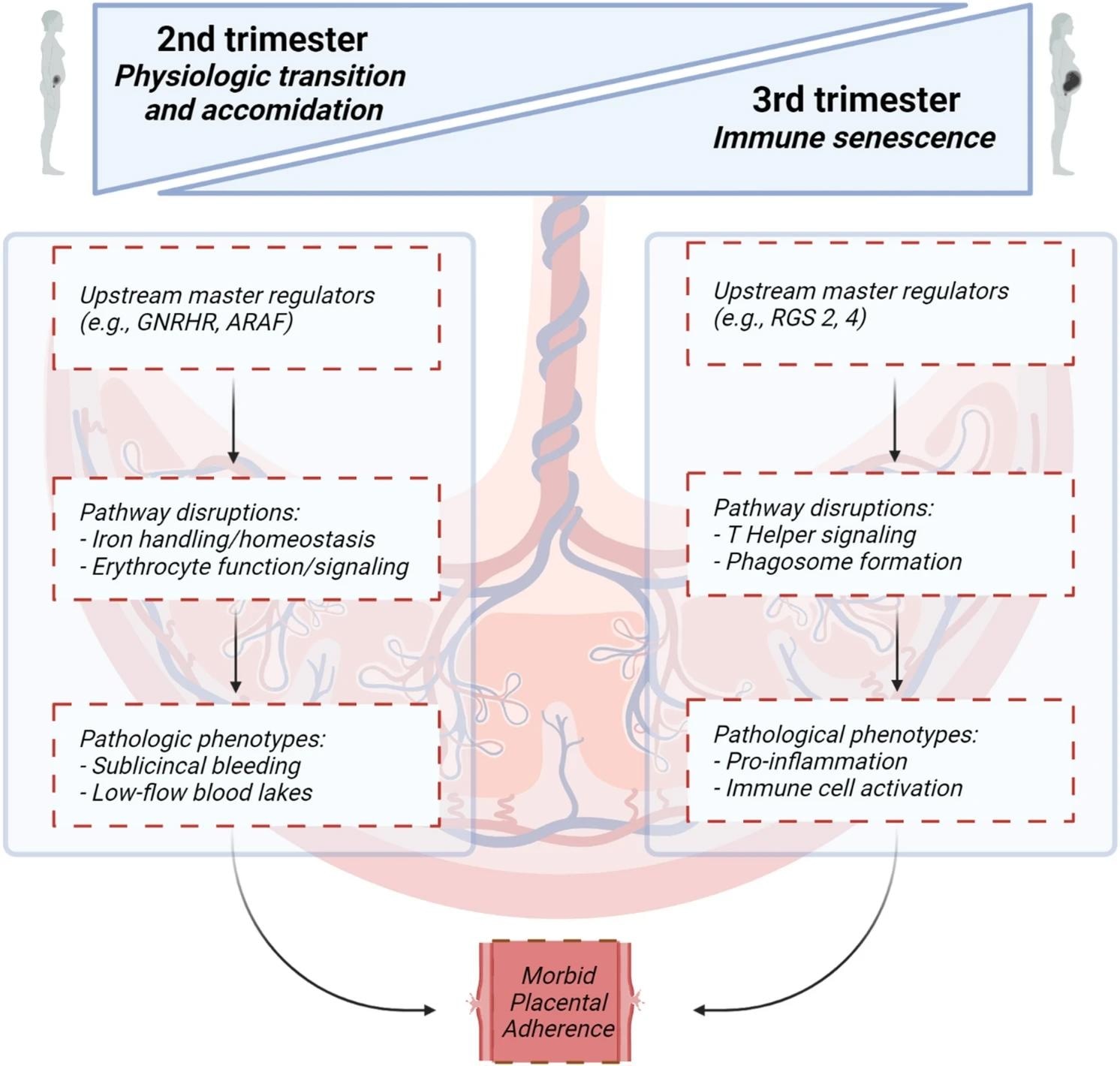 Canonical pathways, upstream regulators and molecular and cellular function analyses proposed in the second and third trimester leading to morbid placental adherence. Figure created with BioRender.com.
Canonical pathways, upstream regulators and molecular and cellular function analyses proposed in the second and third trimester leading to morbid placental adherence. Figure created with BioRender.com.
Proteomic changes in PAS in the second trimester yielded an overrepresentation of canonical pathways, including erythropoietin and iron homeostasis signaling. In addition, cellular and molecular functions around erythrocyte function and iron handling were in agreement with canonical erythropoietin and iron homeostasis signaling pathways revealed by IPA.
Similarly, IPA revealed a significant overrepresentation of upstream regulators, canonical pathways, and cellular and molecular functions in the third trimester. Proteomic changes in PAS in the third trimester revealed a significant overrepresentation of extracellular and immune signaling pathways, particularly those involving interleukin-15 (IL-15).
Cellular and molecular functional analyses identified 24 select annotated functions with significant overrepresentation in the third trimester. However, there was some agreement between canonical pathways and cellular and molecular functions, particularly around cytoskeletal/cellular growth and immune signaling functions.
Conclusions
In summary, the team identified five and four CMP proteins that classify PAS in the second and third trimesters, respectively. Notably, the findings suggested a higher predictive value of markers in the second than in the third trimester. Further analyses revealed an overrepresentation of oxygenation- and blood-cell function-related processes in the second trimester and abnormal immune cell and IL-15 signaling in the third trimester.