Early SARS-CoV-2 Omicron lineage variants have mutated, resulting in divergent lineages that drove the 2022 coronavirus disease 2019 (COVID-19) pandemic. As a result, the U.S. Food and Drug Administration (FDA) authorized bivalent mRNA vaccines designed to widen protection against current and future variants in August 2022, and the U.S. Centers for Disease Control and Prevention (CDC) recommended their use in September 2022. However, the effect of bivalent vaccination on the production of neutralizing antibodies against SARS-CoV-2 homologous Omicron BA.4/BA.5 viruses and emerging heterologous viruses needs extensive evaluation.
 Study: Bivalent mRNA vaccine improves antibody-mediated neutralization of many SARS-CoV-2 Omicron lineage variants. Image Credit: FOTOGRIN / Shutterstock
Study: Bivalent mRNA vaccine improves antibody-mediated neutralization of many SARS-CoV-2 Omicron lineage variants. Image Credit: FOTOGRIN / Shutterstock

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the present study, the researchers examined the neutralization activity of serum samples obtained after the third or fourth COVID-19 vaccination dose against ten prevalent or recent SARS-CoV-2 Omicron lineage viruses, including BA.2.75, BA.2.75.2, BA.1, BA.2, BA.5, BQ.1, BQ.1.1, XBB, XBB.1, and BN.1.
The team obtained serum samples from vaccine recipients two to six weeks after being vaccinated with the third dose or two to seven weeks after vaccination with the fourth dosage of a bivalent booster mRNA vaccine. Using Meso Scale Discovery (MSD) assays, the total immunoglobulin (Ig)-G antibody response was compared between post-third vaccine dose sera and post-fourth vaccine dose sera in response to representative spike proteins associated with the progenitor index virus (614D) and SARS-CoV-2 Alpha, Delta, Beta, and Omicron BA.1, BA.5, BA.2, and BA.2.75 lineages.
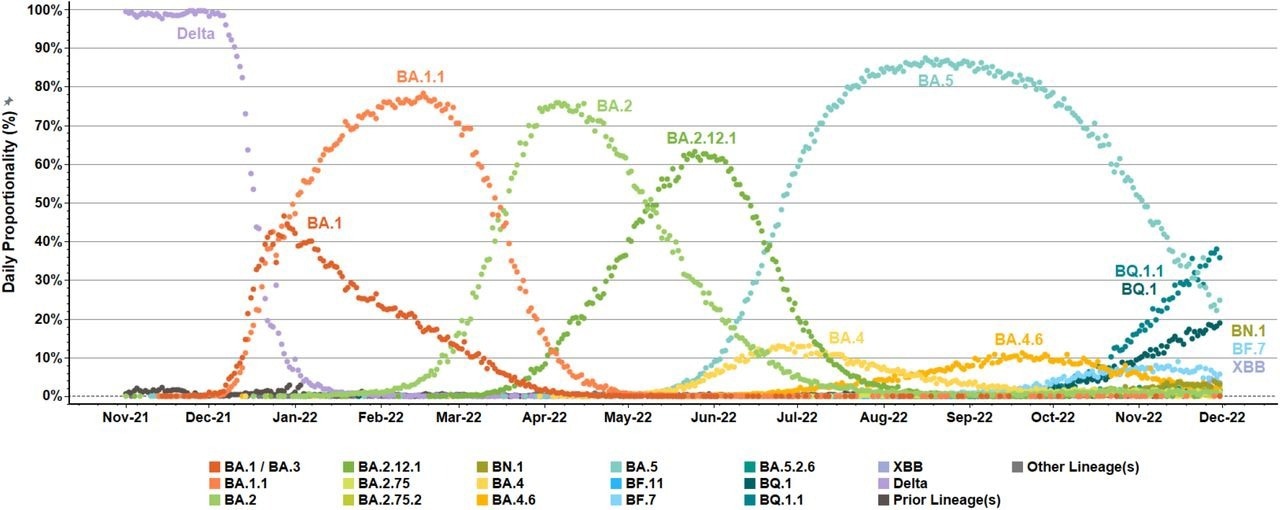
Emergence and diversification of SARS-CoV-2 Omicron variants and lineages in US surveillance networks. Daily, percent proportionalities (dot icons, 0% to 100%) were summarized for detected variant/lineage populations within US National SARS-CoV-2 Strain Surveillance (NS3) and baseline surveillance initiatives from November 1, 2021, to November 30, 2022. Reported sequences were aggregated and color-coded based on attributed Pango lineages. Delta (B.1.617.2) variant preceded the emergence and expansion of Omicron (B.1.1.529, BA) variant within US surveillance networks. Several tracked Omicron (BA) lineages (e.g., BA.2.75, BA.2.75.2, BF.11, and BA.5.2.6) were detected at low levels (≤10%, labels not shown). “Prior lineages” included additional A and B lineages with limited detection. “Other lineages” consolidated the few specimens with no assigned Pango nomenclature or non-XBB X lineage.
To assess the neutralizing effectiveness of post-third vaccination and post-bivalent vaccination dosage sera against major Omicron lineage viruses, the team created lineage-specific spike mutations corresponding to the Omicron BA.1 spike background, giving rise to reporter viruses related to 10 Omicron lineage viruses. To precisely investigate the impact of distinct spike domains in antibody evasion, the lineages BA.1, BA.2, BA.4, and BA.5 were selected, and their receptor-binding domain (RBD) and non-RBD spike variants were isolated. Pooled post-second dose sera and pooled post-third dosage sera were used to examine all the viruses.
Results
After the third vaccine dosage, the anti-spike binding activity decreased minimally for SARS-CoV-2 Alpha, Beta, and Delta, with fold decreases of 1.2, 1.7, and 1.4 concerning the 614D standard, respectively. With fold-change reductions of 4.2, 3.8, 3.7, and 5.6, respectively, BA.1, BA.5, BA.2, and BA.2.75 exhibited the most significant alterations. Compared to the post-third dose serum samples, the post-bivalent vaccination serum had slightly greater activity against all the viral spike antigens. In the MSD test, however, the breadth/specificity of these two sera was comparable, as the fold changes with respect to the 614D spike among B.A.1, BA.5, BA.2, and BA.2.75 observed post-third dose and post-bivalent sera were comparable.
The sera from the third dosage exhibited significant neutralizing activity in response to the 614D reference virus, moderate activity in response to the BA.1, BA.2, BA.5, and BA.2.75 strains, and poor activity against the BA.2.75.2, BQ.1, BQ.1.1, BN.1, XBB, and XBB.1 strains. Compared to the 614D nAb titers, the BA.1, BA.5, BA.2, and BA.2.75 nAb titers were reduced by five, eight, 20, and 12 times, respectively. Compared to 614D, the titers of the BA.2.75-2 and BN.1 lineages fell by 127 and 64 times, respectively. Furthermore, in comparison to 614D, the titers of BQ.1 and BQ.1.1 lineages declined by 190 and 288 times, respectively. XBB and XBB.1 had the most significant degree of escape, with 324 and 371 times reductions in nAb titers compared to 614D.
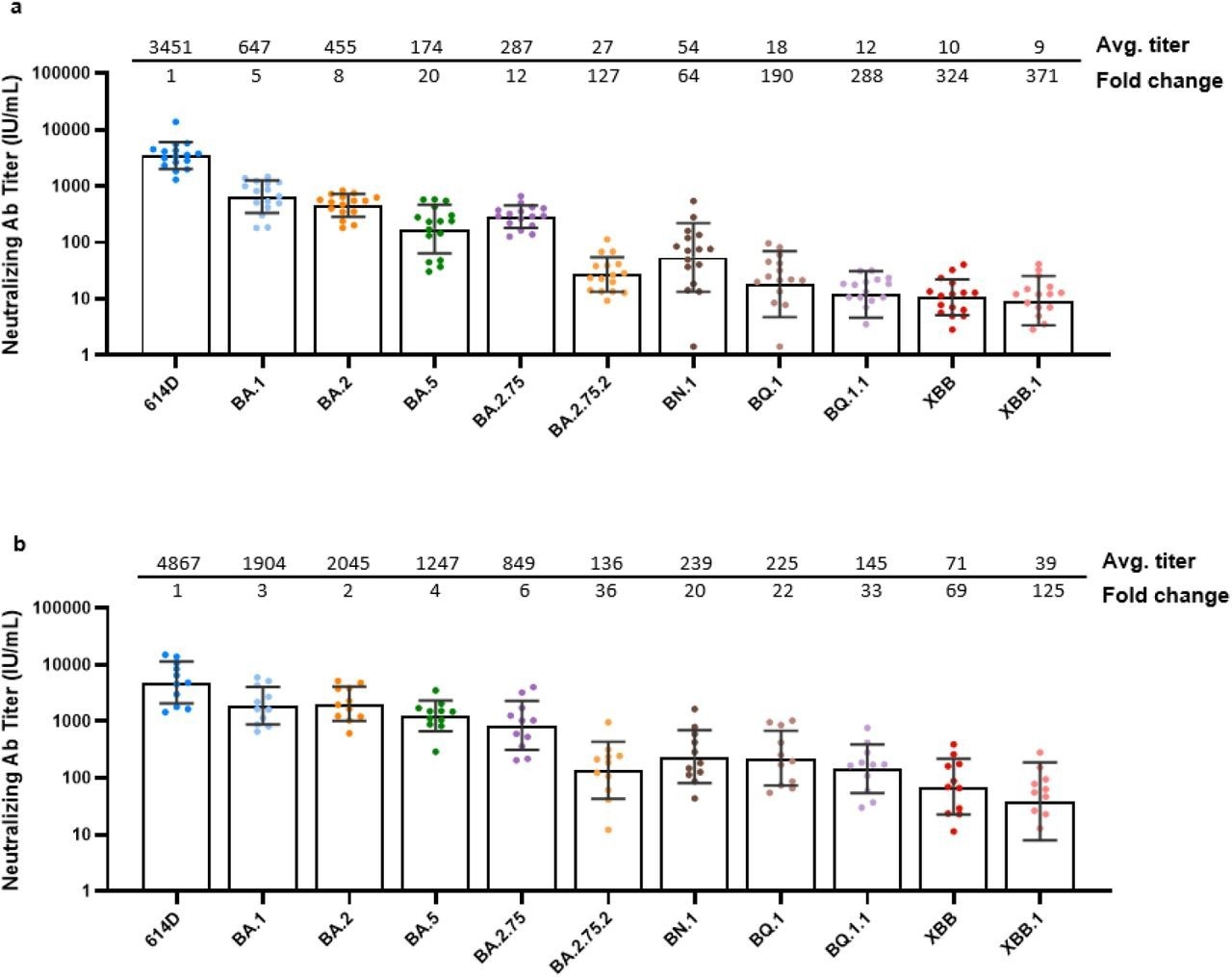
Neutralization activity of post-third (monovalent) dose sera and post-fourth (bivalent) dose sera against Omicron lineages. Each dot represents the neutralizing antibody titer (IU/mL) for each serum-virus pair. For statistical analysis, a two-tailed Wilcoxon matched-pairs signed-rank test was performed by comparing each variant with 614D. Test statistics and P value are summarized in Supplementary Table 2. The geometric mean of neutralizing antibody titer with geometric standard deviation and average fold-change with respect to the 614D reference virus are displayed across the top of each graph for: a, post-third dose sera (8 Pfizer-BioNTech BNT162b2 mRNA vaccine and 8 Moderna mRNA-1273 vaccine biologically independent post-third dose sera examined over 11 viruses); and b, post-fourth dose bivalent vaccine sera (N = 11 biologically independent post-fourth dose bivalent vaccine sera examined over 11 viruses). Average fold-change was calculated as the individual ratios of the geometric mean relative to 614D. Average neutralizing antibody titers in both sera for all lineages differ significantly (P < 0.001) from 614D (Supplementary Table 2). Virus lineage names are displayed across the bottom of each graph.
With respect to the post-third vaccine dose sera, the neutralizing activity noted in response to the 614D reference rose by 1.4 times in the post-bivalent vaccination sera; however, it increased considerably against other Omicron lineages. Post-bivalent vaccination sera were 7.1 times more effective at BA.5 neutralization and 2.9 to 5.1 times more effective at the neutralization of heterologous antigens like BA.1, BA.2, BA.2.75, BA.2.75.2, and BN.1 in comparison to the post-third vaccine dose sera. The more considerable rise of BA.5 lineage viruses compared to BA.1/BA.2 lineage viruses suggested that the bivalent mRNA vaccination induced BA.5 lineage-specific antibodies.
In comparison to the 614D virus, post-second dose and post-third dose titers reductions for the BA.1 were 45- and eight-fold, BA.2 were 38- and 11-fold, BA.5 were 29- and 10-fold and XBB.1 were over 192- and 289-fold. Viruses bearing only RBD mutations were related with post-second and post-third dose titer reductions of one- and one-fold for BA.1, three- and four-fold for BA.2, seven- and eight-fold for BA.5, and 38- and 17-fold for XBB.1. Removing the RBD mutations restored the virus neutralizing titers to values close to 614D in both post-second and post-third serum samples.
Conclusion
The study findings showed that the neutralizing titers improved by 7.1-fold against SARS-CoV-2 Omicron BA.5 lineage and 12-fold against the BQ.1 and BQ.1.1 lineages between the post-third dose monovalent and post-fourth dose bivalent vaccine sera. This indicated that the improved neutralizing titers caused higher vaccine efficiency after bivalent vaccination. Despite the fact that the variables of protection against SARS-CoV-2 must be defined in total, they may be explored further based on additional data from the investigations of the efficacy of the COVID-19 vaccine.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Bivalent mRNA vaccine improves antibody-mediated neutralization of many SARS-CoV-2 Omicron lineage variants, Nannan Jiang, Li Wang, Masato Hatta, Chenchen Feng, Michael Currier, Xudong Lin, Jaber Hossain, Dan Cui, Brian R. Mann, Nicolas A. Kovacs, Wei Wang, Ginger Atteberry, Malania Wilson, Reina Chau, Kristine A. Lacek, Clinton R. Paden, Norman Hassell, Benjamin Rambo-Martin, John R. Barnes, Rebecca J. Kondor, Wesley H. Self, Jillian P. Rhoads, Adrienne Baughman, James D. Chappell, Nathan I. Shapiro, Kevin W. Gibbs, David N. Hager, Adam S. Lauring, Diya Surie, Meredith L. McMorrow, Natalie J. Thornburg, David E. Wentworth, Bin Zhou, bioRxiv, DOI: https://doi.org/10.1101/2023.01.08.523127, https://www.biorxiv.org/content/10.1101/2023.01.08.523127v1