Waltham, MA - The U.S. Food and Drug Administration (FDA) has cleared Nova Primary as a blood glucose reference analyzer. Nova Primary fills the need for a new glucose reference analyzer to replace the discontinued YSI STAT PLUS 2300 Glucose and L-Lactate analyzer (YSI, Inc., Yellow Springs, OH).
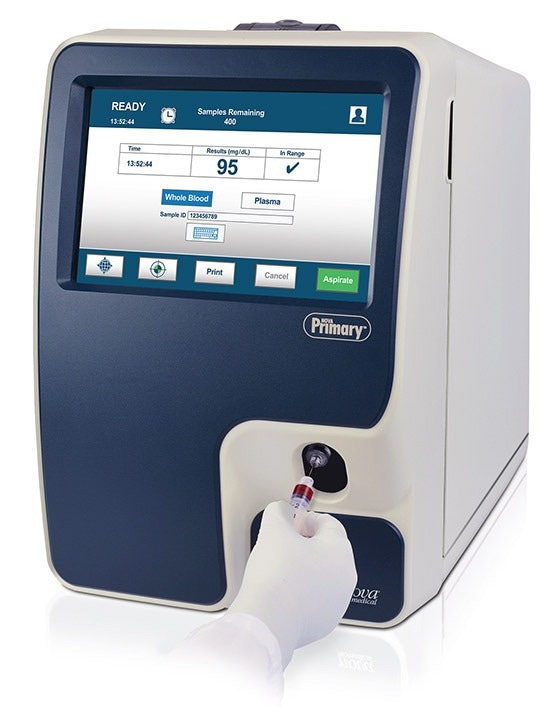
Image credit: Nova Biomedical
Manufacturers of blood glucose measuring devices and clinical diabetes researchers have relied on the YSI 2300 as a reference and correlation analyzer. However, YSI, Inc. no longer supports the analyzer, and its discontinuation has left a critical industry void. The Nova Primary analyzer fills that need. With today’s FDA clearance, Nova Primary is now available in the U.S. and worldwide. According to Matthew McRae, Nova Sales Product Line Manager, “We at Nova Biomedical are pleased to offer the Nova Primary, an accurate, easy-to-use and modernized blood glucose reference analyzer for glucose device manufacturers and clinical diabetes researchers to replace the discontinued YSI Stat Plus glucose analyzer.”
Like the YSI 2300, Nova Primary uses a single, reusable glucose electrochemical sensor based on glucose oxidase and has a measurement range of 20-900 mg/dL. It uses a small, 25 microliter venous whole blood or plasma sample which is internally diluted as in the YSI. Results are available in approximately 2 minutes. Nova Primary’s large color touchscreen display and intuitive, icon-based graphical user interface make it very straightforward to use. A single calibrator pack uses RFID data management to monitor the pack expiration date and number of samples remaining, eliminating the need for separate reagent bottles and daily monitoring of their levels. In clinical laboratory studies using venous whole blood and plasma, the Nova Primary demonstrated excellent correlation to the YSI 2300 and is traceable to NIST glucose standards. The Nova Primary uses a comma delimited (.csv) format data output that can be downloaded via the integral USB data port. The analyzer measures 17 in x 11 in x 18 in (44 cm x 28 cm x 46 cm).