During the 1970s and 1980s, the median age of mpox infection reported in the United States was between four and five years old. However, in recent decades, the median age has risen. In the 1980s, pediatric mpox was associated with a death rate of up to 10%. Clinical microbiology testing is facing significant hurdles when diagnosing infectious illnesses in a low-risk group. The most troubling challenge is the possibility of reporting false positive test results because of poor positive predictive value in settings with low prevalence.
 Study: Clinical Testing of Pediatric Mpox Specimens: Unique Features and Challenges in a Low Prevalence Population. Image Credit: Dotted Yeti / Shutterstock
Study: Clinical Testing of Pediatric Mpox Specimens: Unique Features and Challenges in a Low Prevalence Population. Image Credit: Dotted Yeti / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
About the study
In the present study, researchers retrospectively evaluated results for pediatric mpox testing.
The team performed mpox virus deoxyribonucleic acid (DNA) detection. Specimens were identified as obtained from genital regions if they involved specific terms. The samples were classified as "detected" if the cycle threshold (Ct) was less than 34, "not detected" if Ct was more than 50, or "inconclusive" if Ct was between 34 and 50. Furthermore, specimens that underwent repeated testing were classed as: detected if Ct was less than 50 for both the replicates, not detected if both replicates had a Ct over 50 and inconclusive if one replicate had Ct less than 50 while the other had Ct more than 50.
Patient cases were categorized as "positive," "false positive," and "inconclusive." An individual with a minimum of one identified specimen was categorized as a positive case. An individual was defined as having an inconclusive case if they had a minimum of one inconclusive sample and no detected samples. False positives were classified when the ordering provider presented a clinical history inconsistent with mpox while no repeat sample testing was discovered.
Results
During the study, 13.4% of all mpox tests were conducted on pediatric specimens. The proportion of children to adults in the cohort was comparable at 13.6%. The pediatric cohort consisted of 56.8% males, 42.7% females, and 0.5% persons of unknown sex, whereas the adult cohort was composed of 66.8% males, 31.0% females, and 2.2% persons of unknown sex. The distribution of gender varied considerably among the two age cohorts. In the pediatric group, 9.0% of samples were acquired from genital sources, and 19.6% of adult samples were obtained from genital regions.
Comparing the adult and pediatric cohorts revealed positive rates of 22.3% and 1.3%, respectively. Furthermore, the positivity rate reported by the female and male pediatric groups was 1.1% and 1.4%, respectively. In the adult group, the female positivity rate was 3.2%, and the male positivity rate was 31.1%. The median Ct value of mpox-positive pediatric specimens was 25.2, and that of mpox-positive adult specimens was 22.2. However, this difference did not show statistical significance.
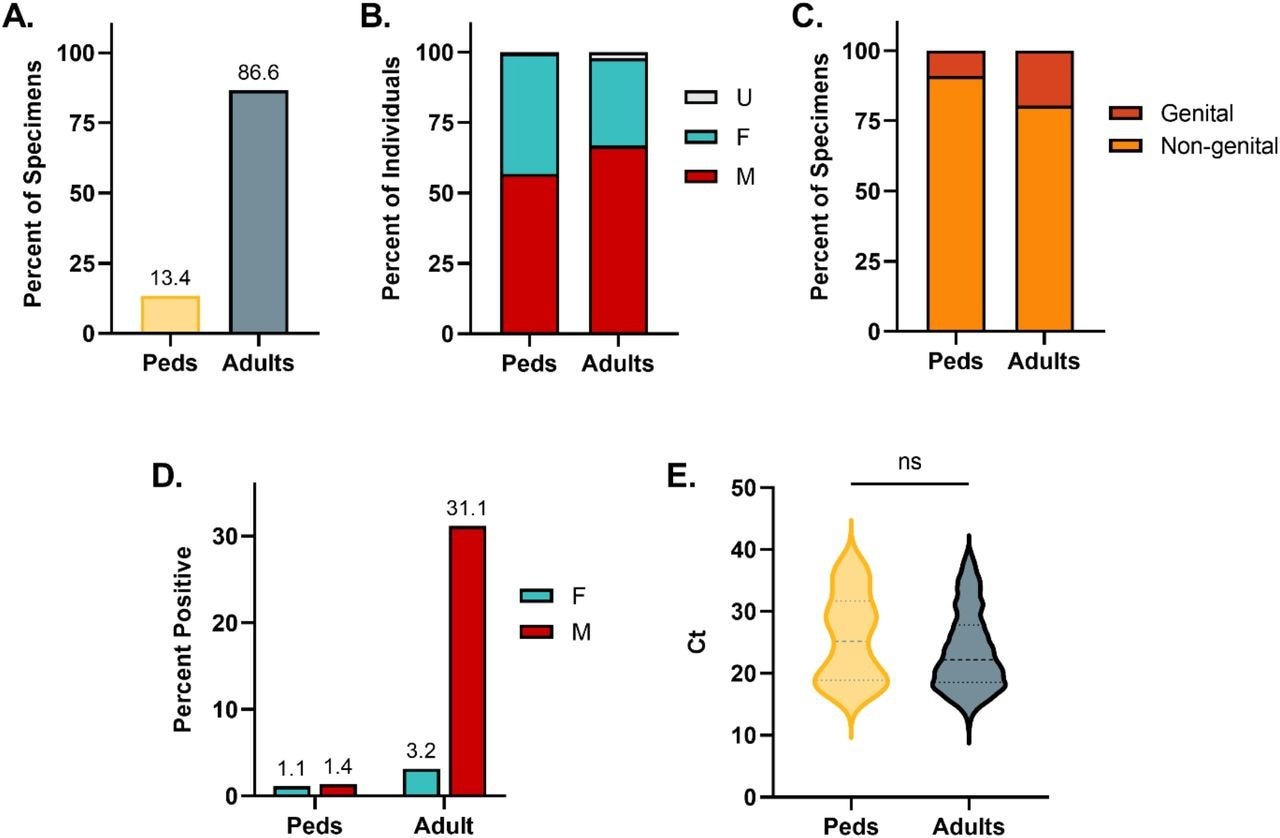
Demographic and Clinical Data for Mpox Specimens from Pediatric and Adult Individuals. (A) Percent of total specimen volume. (B) Percent of female, male, and unknown sex. (C) Percent of specimens obtained from genital or non-genital sources. (D) Positivity rate of pediatric and adult specimens stratified by sex. (E) Comparison of Ct values between pediatric and adult specimens positive for mpox
The pediatric cohort reported a median age of 6 ± 5.4 years. The patients tested most often were one-year-olds (10.1%), followed by two-year-olds (9.2%), three-year-olds (8.2%), and 17-year-olds (7.3%). Notably, only 3.5% of the tested patients were 11 years old. The age distribution of mpox infections exhibited bimodal peaks, with the highest proportion of positive cases occurring in 17-year-olds (36.8%), followed by patients aged less than one year (26.8%). One positive case was detected in cohorts of children aged one, two, six, eight, nine, 14, and 16 years. The rate of mpox positivity among children aged less than one year was 7.8%, one year was 0.9%, two years was 0.7%, six years was 1.1%, eight years was 1.4%, nine years was 1.9%, 14 years was 1.6%, 16 years was 1.3%, and 17 years was 6.4%. Interestingly, females were diagnosed with mpox at an average age of 4,5 years while males displayed an average age of 11.6 years.
Almost 73% of the 26 persons for whom MPXV was amplified a minimum of once from a specimen were confirmed to be mpox-positive, 19% were inconclusive, and 8% were false-positive for mpox. The sensitivity and specificity of the test were 100% and 99.9, respectively. Furthermore, the team noted that two instances from the study represented false positive outcomes. In both instances, the late Ct values of 35.2 and 39.1 did not reappear after re-extraction and amplification.
Conclusion
The study findings showed that a minority of pediatric individuals were tested for mpox. The overall positivity rate is significantly lower among pediatric persons than in adult males. The majority of pediatric samples were from non-genital regions and were obtained from females. The age groups with the highest mpox risk appear to be those aged less than one year and 17 years. Significant numbers of early mpox detections are characterized by inconclusive and false-positive results. The researchers advise that careful clinical correlation and repeat testing of patient samples should be considered, especially within the pediatric age groups.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources