
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Important considerations for new COVID-19 vaccines
The original coronavirus disease 2019 (COVID-19) vaccines were effective against the ancestral SARS-CoV-2 strain and had successfully reduced disease severity by over 90%. However, the waning of vaccine-induced immunity and the emergence of new viral variants with mutations in the spike protein that enables immune evasion have threatened the efficacy of these vaccines.
Thus, there remains an urgent need for more effective booster vaccines and the development of pan-coronavirus or variant-specific vaccines. Furthermore, the protection of immunocompromised individuals continues to remain a challenge.
In April 2022, the World Health Organization (WHO) revised the COVID-19 vaccine target product profile to address the shortcomings of current vaccines in light of emergent SARS-CoV-2 variants. Some desired characteristics for next-generation vaccines include broader protection against different variants, durable vaccine-induced protection, and ease of manufacturing and distribution. The existing messenger ribonucleic acid (mRNA) and viral-vectored vaccines meet only one or the other of these criteria.
About the study
In the present study, researchers designed a protein component vaccine consisting of the SARS-CoV-2 spike protein receptor binding domain (RBD) and sequences adjacent to the RBD, as previous studies have reported higher neutralizing antibody titers from RBD-based COVID-19 vaccines. In addition, the vaccine was also designed to be highly soluble, stable, expressed at high levels, and receptive to purification protocols, all of which are properties that could make its manufacturing and distribution less complicated.
The spike protein sequence of the ancestral SARS-CoV-2, Wuhan Hu-1 strain, was analyzed to determine the vaccine's biophysical, structural, and biochemical properties. Then, the final codon-optimized RBD construct, which spanned the spike residues 316 to 594, was expressed using a secretion vector.
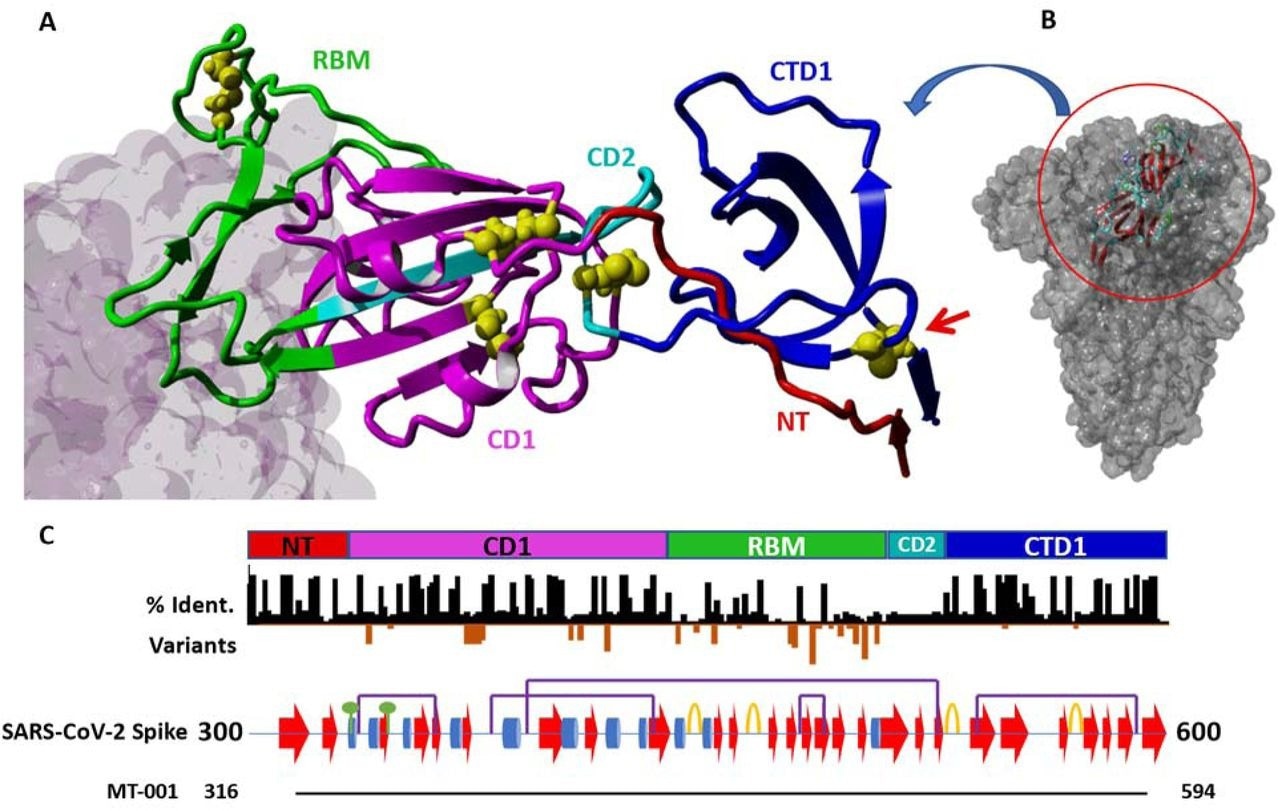
Detail of the SARS-CoV-2 spike protein in the region 300-600 and MT-001 construct design. A) Structure of MT-001 construct derived from PDB IDs 7BYR and 7KNE. The RBD construct is color-coded by annotated blocks of amino acid sequence ("regions," see panel C and ref. 45). Cysteines are shown as yellow balls. The ligand of the RBM, ACE2 (from 7KNE), is shown as a gray molecular surface (left). B) The MT-001 construct (ribbon) is shown in the context of the full-length spike trimer (space-filling model). C) Schematic of the regions shown in (A). Top: Color-coded region key for the MT-001 construct in (A). NT: N-terminal region (residues 316-332, red); CD1: "core domain 1" region (333-436, magenta); RBM: receptor binding motif (437-508, green); CD2: "core domain 2" region (509-527, cyan); CTD1: "C-terminal domain 1" region (528-594, blue). A red arrow indicates the 538-590 disulfide bond that stabilizes CTD1. Middle: Black bars - Sequence identity per residue between SARS-CoV-2 spike and representative members of the coronavirus superfamily, demonstrating highly conserved regions N- and C-terminal to the RBM (STable 1). Orange bars - Sites of and frequency of mutations in characterized SARS-CoV-2 variants (3). Lower: Schematic showing the secondary structure and post-translational modifications in the region from residues 300-600 in the SARS-CoV-2 spike protein. Alpha helices are shown as blue cylinders, beta sheets as red arrows, and turns as orange loops. Disulfide bonds are denoted with purple bridges and N-linked glycosylation sites with green circles. Bottom: Alignment of the MT-001 construct with the visualized region.
The antigenicity and durability of the vaccine were tested on BALB/cJ mice and Syrian golden hamsters. Mice were intramuscularly immunized with different concentrations of the MT-001 vaccine and boosted on day 21.
Serum samples were collected three days before vaccination and at different points after immunization. Sandwich enzyme-linked immunosorbent assay (ELISA) was used to determine anti-RBD IgG levels.
One group of Syrian golden hamsters was intramuscularly inoculated with the adjuvanted MT-001 vaccine, while another that served as the control group was inoculated with phosphate-buffered saline (PBS). Both groups were administered a booster dose of the MT-001 vaccine or PBS on day 21. Blood samples were collected before vaccination and at two, three, four, five, and six weeks after vaccination.
On day 42, all hamsters were challenged with the USA-WA-1 strain of SARS-CoV-2, after which all animals were weighed each day and eventually euthanized on the fourth day to obtain blood and lungs for viral load assessment.
A quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay was performed on total ribonucleic acid (RNA) extracted from the lungs to determine their viral load, whereas a SARS-CoV-2 neutralization assay was performed on the serum samples. Additionally, lung sections were stained with hematoxylin-eosin for histopathological analysis.
Study findings
Two preliminary doses of the MT-001 vaccine in BALB/cJ mice elicited comparable anti-RBD IgG titers as the Pfizer/BioNTech and Moderna mRNA vaccines. Furthermore, the adjuvanted MT-001 vaccine also showed a balance in the type 1 T helper cell (Th1) and type 2 T helper cell (Th2) responses.
Syrian golden hamsters immunized with the adjuvanted MT-001 vaccine and challenged with SARS-CoV-2 after six weeks had undetectable viral loads in the lung tissue after four days.
The half-maximal effective concentrations of the IgG titers against the SARS-CoV-2 spike protein RBD in the sera of immunized mice were in the 105–106 range, even 12 months after vaccination.
The addition of the toll-like receptor-9 (TLR-9) agonist CpG oligonucleotide (ODN) 1826 significantly increased anti-spike IgG titers. The inclusion of CpG ODN 1826 also produced a broadly cross-reactive immune response against the SARS-CoV-2 Delta and Omicron variants, with neutralization antibody levels against the Omicron BA.1 variant comparable to those elicited by the variant-specific mRNA vaccine mRNA-1273.529.
Conclusions
The study findings demonstrate that the SARS-CoV-2 protein component vaccine MT-001 is a promising candidate for a next-generation vaccine against COVID-19. MT-001 elicited high and durable anti-spike IgG titers in mice and hamster models, as well as broadly cross-reactive immune responses against SARS-CoV-2 variants when combined with CpG ODN 1826.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Campbell, E., Dobkin, J., Osorio, L., et al. (2023). A SARS-CoV-2 vaccine designed for manufacturability results in unexpected potency and non-waning humoral response. bioRxiv. doi:10.1101/2023.02.06.527376. https://www.biorxiv.org/content/10.1101/2023.02.06.527376v1
- Peer reviewed and published scientific report.
Campbell, Elliot, Julie Dobkin, Louis J. Osorio, Afsal Kolloli, Santhamani Ramasamy, Ranjeet Kumar, Derek B. Sant’Angelo, Selvakumar Subbian, Lisa K. Denzin, and Stephen Anderson. 2023. “A SARS-CoV-2 Vaccine Designed for Manufacturability Results in Unexpected Potency and Non-Waning Humoral Response.” Vaccines 11 (4): 832. https://doi.org/10.3390/vaccines11040832. https://www.mdpi.com/2076-393X/11/4/832.