The influenza season marks the beginning of a time of illness, sometimes serious or even fatal, in much of the world. For this reason, flu shots are recommended, especially for individuals with underlying diseases such as chronic hepatitis B (CHB) infection, since they are at higher risk for adverse outcomes. The same is true of the coronavirus disease 2019 (COVID-19) vaccines, intended to prevent severe disease following infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
A recent study explored the immune responses to these vaccines in chronic HBV infection.
Every year, about 3-5 million people fall severely ill with the flu, with ~300,000 to 650,000 deaths occurring annually due to respiratory symptoms following the flu. In recent years, the advent of COVID-19 has increased the danger from the flu, as both often occur together, increasing the severity of disease.
People with cancer or weakened immunity, or underlying inflammation, including those with obesity, are strongly recommended to take flu shots as they respond less well to either of these viral infections. Chronic infections also seem to have the same effect.
CHB is a troubling and widespread illness, more so in east Asia, leading to long-term liver damage, weakened immunity, and chronic inflammation. The current study, published in the journal Immunity, Inflammation and Disease, explored immune responses to the inactivated flu and COVID-19 vaccines in this group of patients. The research is a follow-up to earlier work that showed lower levels of anti-SARS-CoV-2 antibodies at one month from inactivated COVID-19 vaccine administration compared to healthy controls.
The study included 57 CHB patients with 19 controls. All were receiving flu shots comprising inactivated influenza virus particles. They were evaluated for anti-influenza immunoglobulin (Ig) G antibodies to the H1N1, H3N2, and B antigens, along with T follicular helper cells (Tfh).
These Tfh cells are CD4 T cells that promote B cell maturation and antibody production. They may be involved in the antibody response after a flu shot, but no significant differences have been found between patients and controls in Tfh cell frequencies.
Of the group of CHB patients, eight were vaccinated against COVID-19, with inactivated virus vaccines, during the first year of follow-up after the flu shots. Earlier research suggests that the effects of influenza vaccination might blunt the immune response to SARS-CoV-2, reducing the severity of illness and mortality due to the latter. Therefore, the scientists measured anti-SARS-CoV-2 antibodies in these patients.
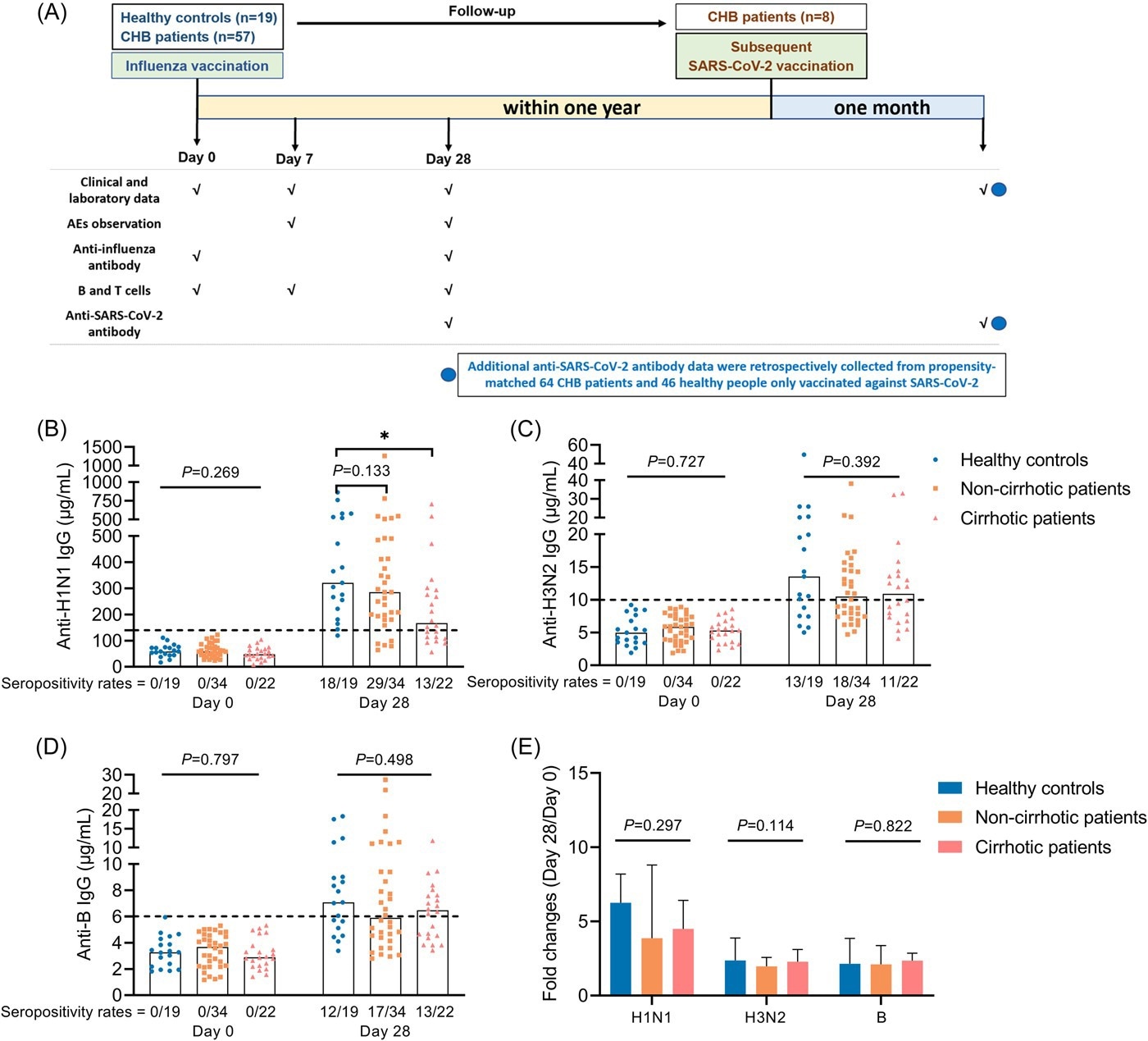
Study flow chart and antibody response to influenza vaccine. (A) Study flow chart. (B–D) Anti-H1N1 IgG (B), anti-H3N2 IgG (C), and anti-B IgG (D) levels in HCs, cirrhotic and non-cirrhotic patients with CHB at day 0 and 28. (E) Fold changes of antibody levels (day 28/day 0) in HCs, cirrhotic and non-cirrhotic patients with CHB. Kruskal–Wallis test was used for comparison. Bonferroni's correction was used, and adjusted p values were represented in this figure. Top of all bars represents median value. *p < .05. Day 0 represents the influenza vaccination day, day 7 represents 7 days after influenza vaccination, and day 28 represents 28 days after influenza vaccination. HCs, healthy controls; IgG, immunoglobulin G.
What did the study show?
The results showed that anti-influenza antibodies were not found at baseline in any participant. On day 28, a similar proportion of participants had seroconverted, but antibodies were higher in controls than in CHB patients at 28 days from the flu shot. The levels of anti-H1N1 IgG were markedly lower in CHB patients with cirrhosis on day 28.
Also, anti-H3N2 IgG levels were found to change less in CHB patients vs. controls, while the two other antibodies showed similar fold changes in both categories.
The levels of anti-H1N1 IgG on day 28 fell in concordance with antibody-secreting cell (ASC) frequencies as measured on day 7. These cells peaked on day 7 but fell steeply by day 28 in all participants. ASC frequency was much lower in CHB patients and more so in those with cirrhosis.
ASC frequency and the anti-H1N1 IgG level were also lower in patients with higher levels of hepatitis B virus (HBV) surface antigens (HbsAg). However, ASC frequency was not correlated with anti-H1N1 IgG levels on day 28, suggesting other reasons for the difference in the former measure.
Patients who had already taken the flu shots responded with higher levels of anti-SARS-CoV-2 antibodies following the COVID-19 vaccination compared to those who had not. Seropositivity was similar in both groups, both the anti-RBD (receptor binding domain) and neutralizing antibodies (NAbs) to SARS-CoV-2.
A history of influenza vaccination was positively associated with both anti-RBD IgG and NAb levels following two doses of an inactivated COVID-19 vaccine within the following year. These vaccines also appeared to be safe in CHB patients.
What are the implications?
Patients with CHB responded less well to the inactivated influenza vaccine, and this response was worse in those with cirrhosis. However, patients with a history of taking these shots within a year of the inactivated COVID-19 vaccine seemed to show higher antibody responses against SARS-CoV-2.
The flu shot may affect the immune response to the inactivated COVID-19 vaccine. This response, perhaps due to trained immunity, “might partially explain why influenza vaccination reduces SARS-CoV-2 infection, severe symptoms, and death.” More extensive studies are required to validate these findings and to understand the mechanism at work behind them.