Their research methodology comprised a 72-week, double-blind, randomized, 1:1, placebo-controlled trial on adults whose 12-week lifestyle interventions resulted in weight losses of 5% or greater.
The findings revealed that tirzepatide promoted additional weight loss in these patients, with mild to moderate gastrointestinal disorders being the most common side effect.
 Image Credit: Stock-Asso / Shutterstock
Image Credit: Stock-Asso / Shutterstock
Obesity and lifestyle intervention limitations
Obesity is a worldwide condition, with over 1 billion patients suffering from the non-transmissible ailment. Attributable to a combination of genetic, lifestyle, and socioeconomic factors, overweight and obesity rates are on the rise, with the World Health Organization estimating an additional 167 million patients by 2025. Overweight and obesity are accompanied by a host of comorbidities, including diabetes, cardiometabolic risk factors like high blood pressure, osteoarthritis, and even cancer.
The gold standard in treating these conditions is intensive lifestyle interventions involving a combination of a reduction in calorific intake, routine intensive physical activity, and frequent behavioral counseling by trained professionals. Generally successful at its onset, lifestyle interventions have two main limitations – 1. Less than 20% of treated individuals present the 15% or greater weight losses necessary to benefit from significantly reduced risk of comorbidities, and 2. In the year following interventions, most patients experience a relapse in weight gain, mainly due to persistent metabolic adaptations, including increases in hunger hormones, decreases in satiety hormones, and reductions in overall energy expenditure.
In recent years, research has revealed incretin-based antiobesity medications as having the potential to surmount these limitations - Semaglutide 2.4 mg, a glucagon-like peptide-1 (GLP-1) receptor agonist, has been shown to result in a 15% reduction in baseline body weight in the two years following intensive lifestyle interventions. Its mode of action involves modifications of pathways governing the signaling of hunger and satiety in select neural regions, thereby preventing abnormal calorific intake.
Tirzepatide is a medication currently approved for the treatment of type 2 diabetes mellitus (T2D). Administered via a subcutaneous injection, this single molecule combined the properties of semaglutide (GLP-1 receptor agonist) while providing the additional benefit of being a glucose-dependent insulinotropic polypeptide. Tirzepatide thereby provides synergistic antiobesity effects of reduced appetite, reduced energy intake, and improved metabolic function. While approved by the United States of America (USA), the European Union (EU), and Japan for treating T2D, its use as an antiobesity intervention is still under review, with confounding results between studies.
About the study
In the present study, researchers conducted a long-term (72-weeks), placebo-controlled randomized trial to elucidate the efficacy of tirzepatide following 12 weeks of intensive lifestyle interventions. The study lasted 84 weeks and was conducted in 62 medical research centers across the USA, Brazil, and Argentina. Participants were eligible if they were adults over the age of 18 years and obese (body mass index [BMI] ≥ 30 kg/m2) or overweight (BMI ≥ 27 kg/m2) while also presenting at least one comorbidity of the conditions. Patients with preexisting diabetes mellitus (type 1 or 2) or who had lost more than 5 kg body weight in the three months preceding the study were excluded.
Between 12 April 2021 and 3 September 2021, 972 participants were assessed for eligibility, of which 806 were enrolled. All enrolled participants were subject to 12 weeks of intensive lifestyle interventions (called the ‘lead-in’ period). The lead-in comprised reductions in calorific intake (1200 kcal for women, 1500 kcal for men), engaging in at least 150 minutes of physical activity per week (moderate intensity), and frequent face-to-face counseling sessions (eight sessions over 12 weeks). Participants were advised to complete 3-day long exercise and diet logs before each counseling session.
Participants who presented a weight loss of 5 kg or greater by week 12 were enrolled for the second phase of the study – the 72-week-long trial involving the use of either the maximum tolerated dose (MTD) of tirzepatide (10 – 15 mg) or a placebo. Participants were assigned to either the case or control groups in a 1:1 ratio via computer-generated randomization, and all researchers and participants were blind to treatment assignment. The interventions were administered once weekly as a subcutaneous injection, with a starting dose of 2.5 mg of tirzepatide increased by 2.5 mg every four weeks until MTD was attained.
Study findings
Of the 806 participants enrolled in the study, 579 (71.8%) presented weight loss of 5 kg or greater (mean 6.9% weight reduction) by week 12 and were included in the tirzepatide trial. Most included participants were white (86.0%), female (62.9%), and had a mean age of 45.6 years. Medical characteristics included an average obesity duration of 15.1 years, and 66.1% of participants presented one or more obesity-related comorbidities.
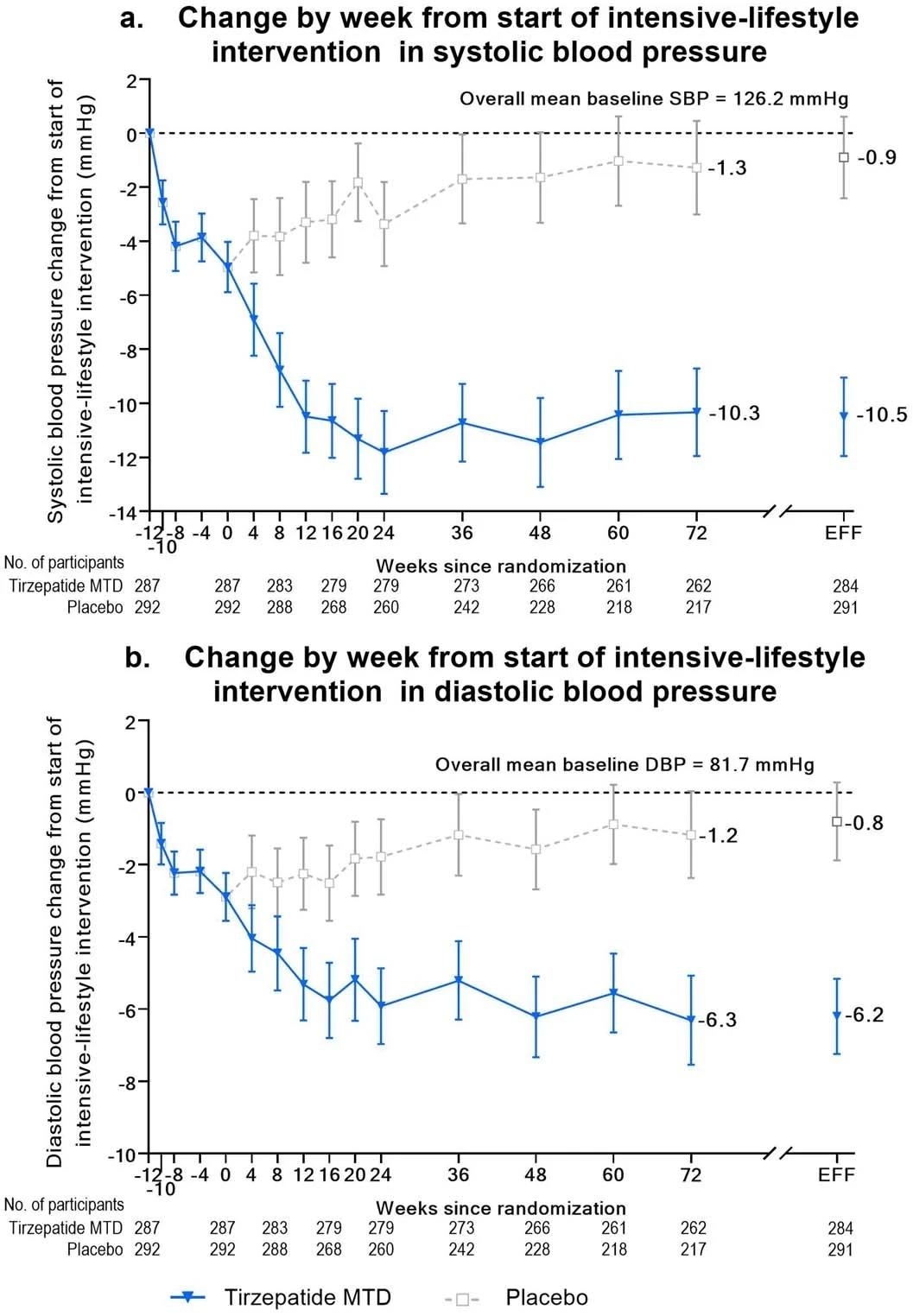
Blood pressure change from start of lead-in period over time. Panel A, mean (95% confidence interval) change from baseline over time in systolic blood pressure from start of intensive-lifestyle intervention lead-in period (week -12) to 72 weeks using observed means. Week 72 estimates for the efficacy estimand (EFF) are also shown. Panel B, mean (95% confidence interval) change from baseline over time in diastolic blood pressure from start of intensive-lifestyle intervention lead-in period (week -12) to 72 weeks using observed means. Week 72 estimates for the efficacy estimand (EFF) are also shown.
Statistical analyses revealed that a greater proportion of participants receiving tirzepatide interventions either lost additional weight or maintained ≥80% of weight lost during the lead-in period compared to the placebo group. These results are highlighted in efficacy statistics, revealing that 98.6% of case participants maintained the ≥80% endpoint versus only 37.8% of the placebo controls.
Case participants experienced an average weight loss of -26.6% at the end of the 84-week-long study, compared to -3.8% in the control cohort. Correspondingly, the average BMI change of the case-cohort was −10.4 kg/m2 compared to –1.4 kg/m2 in the control cohort. Cardiometabolic health outcomes in the case-cohort were significantly improved, most notably in both systolic- and diastolic blood pressure, all fasting lipid levels, glycemic control, and fasting insulin levels.
“In addition, 4.9 and 2.8% of participants in the tirzepatide group compared with 1.0 and 1.7% of participants in the placebo group were reported as having a decrease in intensity of antihypertensive and lipid-lowering medications, respectively. Conversely, 2.4 and 0.3% of participants in the tirzepatide group were reported to have experienced an increase in intensity of antihypertensive and lipid-lowering therapies, respectively, compared with 6.5 and 2.1% of participants in the placebo group.”
Self-reported physical function was observed to be higher in the case-cohort when compared to the control group. However, tirzepatide was not without its shortcomings – 87.1% of treated patients reported treatment-emergent adverse effects, the most common of which were gastrointestinal, including diarrhea, nausea, and constipation. The severity was observed to be mild to moderate.
“Serious adverse events were reported by 31 participants (5.4%) overall. Occurrence was similar in participants treated with tirzepatide (5.9%) and placebo (4.8%). Two deaths (both myocardial infarction) were reported during the study, one in the tirzepatide MTD group and one in the placebo group. Both events were considered not to be related to the study treatment by the investigator.”
Conclusions
The present study aimed to evaluate the efficacy of tirzepatide as an antiobesity medication to promote weight loss and prevent a relapse of weight gain in overweight and obese patients receiving intensive lifestyle interventions. The 84-week-long study revealed that tirzepatide MTD interventions resulted in significantly improved weight loss compared to the placebo control group, corresponding improvements in cardiometabolic function, and reduced obesity-related comorbidities.
Mild to moderate side effects were reported by 87.1% of participants, with serious side effects reported by 5.9%. These side effects were mainly gastrointestinal in nature and may be controlled by additional medication and dietary interventions. Future research investigating optimized, patient-specific dosages instead of MTD might also improve these outcomes and reduce the adverse effects of this intervention.
Journal reference:
- Wadden, T. A., Chao, A. M., Machineni, S., Kushner, R., Ard, J., Srivastava, G., Halpern, B., Zhang, S., Chen, J., Bunck, M. C., Ahmad, N. N., & Forrester, T. (2023). Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nature Medicine, 1-10, DOI – https://doi.org/10.1038/s41591-023-02597-w, https://www.nature.com/articles/s41591-023-02597-w