Medical imaging often facilitates the successful diagnosis and management of cancerous growths. In particular, magnetic resonance imaging (MRI) is extensively used, especially with contrast agents, because of its high resolution.
 Study: Self-folding macromolecular drug carrier for cancer imaging and therapy. Image Credit: S. Singha / Shutterstock.com
Study: Self-folding macromolecular drug carrier for cancer imaging and therapy. Image Credit: S. Singha / Shutterstock.com
What are contrast agents?
Contrast agents (CAs) such as complex paramagnetic metal ions are exploited for their ability to alter the nuclear relaxation rates of hydrogen nuclei. The binding of macromolecules to these complexes can increase relaxivity, thereby producing a slow-tumbling effect.
At the nanoscale, molecules persist in the bloodstream for extended periods and can enter solid tumors without inducing tumor-specific immune-evasive mechanisms. Several types of molecular complexes built on nano-sized molecules have been studied as potential carriers for CAs into tumors.
These nano-sized contrast agents (NCAs) must be properly distributed between the blood and tissue of interest to minimize background noise and obtain the greatest signal-to-noise ratio (S/N). At high concentrations, NCAs persist for longer durations in the bloodstream, thus increasing the risk of widespread fibrosis due to the release of gadolinium ions from the complexes.
Unfortunately, most NCAs currently in use comprise assemblies of several different types of molecules. Below a certain threshold, these micelles or aggregates tend to dissociate, and the outcomes of this event are not yet clear.
This has motivated research into self-folding nanoscale macromolecules that do not have a critical dissociation threshold. Composed of a fatty core with a soluble outer layer, these may also be able to limit the movement of soluble units at the surface of contact. This may subsequently affect molecular relaxation parameters and other functions that can be manipulated to enhance drug delivery and specificity attributes in vivo.
The current study reports on the first-ever self-folding macromolecular drug carrier (SMDC) loaded with a gadolinium (Gd)-1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid (Gd-DOTA).
What did the study show?
The introduction of a novel mechanism into the NCA enhanced the longitudinal relaxation state of its protons, thereby causing it to produce sharper images at much lower loads of gadolinium complexes. The lower load reduces the risk of adverse effects, as the dosage of the CA is minimal.
To construct the SMDC-Gd complex, a copolymer containing fatty, soluble, and CA-loaded segments was first formed. The formation of the copolymer is a crucial step in the process, as it determines whether it will fold upon itself to reduce the number of fatty units in each polymer molecule.
The resulting SMDC has a dense core with a crowded complex environment due to the self-folding property. This increases relaxivity, as internal and segmental movements may be limited around the SMDC-Gd interface.
Reversible addition-fragmentation chain transfer (RAFT) was used to produce several random copolymers of poly (ethylene glycol) methyl ether acrylate (PEGA) and benzyl acrylate (BZA). While BZA is fat-soluble, PEGA is water-soluble.
Size-exclusion chromatography equipped with multi-angle light scattering (SEC-MALS) was combined with transmission electron microscopy (TEM) and small-angle X-ray scattering (SAXS) to identify P7, which was the best candidate for SMDC. This is based on a degree of aggregation (DA) in water above one, which will self-assemble by intermolecular forces, and equal proportions of BZA/PEGA, yielding particles between six and eight nanometers (nm) in water. This favors SMDC formation, even after adding a small amount of Gd/DOTA to the side chain.
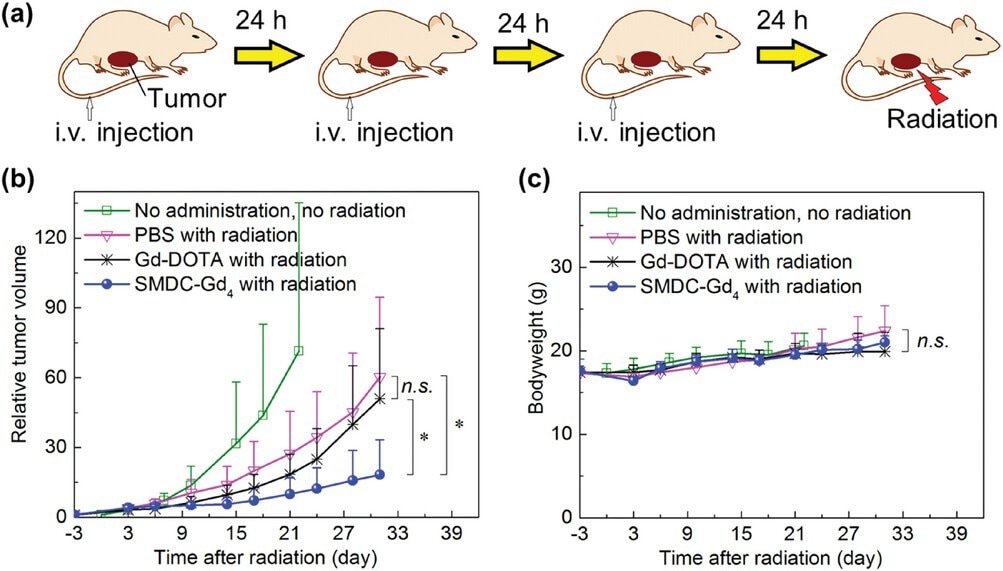
Anti-tumor effect of SMDC-Gd4 in Gd-NCT against CT26 tumor-bearing mice. a) Schematic illustration of the therapeutic regimen for Gd-NCT. Daily injections for three consecutive days were given, followed by thermal neutron irradiation (for 10 min, 5MW, fluence: 2.87 × 1012 to 3.29 × 1012 thermal neutrons cm−2, 5.10 × 1011 to 5.86 × 1011 epithermal neutrons cm−2) directly toward subcutaneous solid tumors 24 h after the last injection. b) Relative tumor volumes in BALB/c mice. SMDC-Gd4 combined with radiation showed a significant anti-tumor effect compared with other groups within 31 days after radiation. Data are shown as mean ± s.d., n = 5, n.s. p ≥ 0.05, ⁎p < 0.05. c) Bodyweight of mice. Data are shown as mean ± s.d., n = 5, n.s. p ≥ 0.05.
This is the first time a drug carrier less than 10 nm in diameter has been constructed through polymer engineering. Importantly, particles 30-100 nm in size are considered optimal for entering and accumulating in solid tumors. This size range favors excretion through urine, as particles can leave the glomerular capillaries. Moreover, these complexes bear negative charges on their surface and do not dissociate below a critical threshold.
Attaching the Gd-DOTA to the polymer enhanced the relaxivity increased beyond the slow tumbling effect; however, a further increase in Gd content was not associated with this effect. The complex retained its favorable properties in the bloodstream, thus indicating that it could be injected intravenously.
An SMDC-Gd formulation termed SMDC-Gd4 was injected into mice bearing colon carcinoma grafts. SMDC-Gd4 accumulated at higher levels in the tumor, unlike Gd-DOTA, which left the bloodstream rapidly and did not accumulate in the tumor.
The self-folding property appears to be responsible for this by forming a PEGylated coating around the SMDC that helps the injected form resist aggregation and protein binding, thereby remaining bioavailable.
SMDC-Gds also exhibited better tumor contrast accumulation over time, with clear images, high resolution, and better contrast enhancement as compared to Gd-DOTA. In fact, the tumor-to-muscle R1 ratio of SMDC-Gd4 increased by 1.36, whereas that of the tumor more than doubled within 24 hours as compared to Gd-DOTA.
This NCA can accumulate within tumors, with the potential for more specific and effective treatment of the tumor using Gd neutron capture therapy. To date, this has not been achieved clinically due to the lack of selectivity in delivering 157Gd into tumors and maintaining it at the appropriate concentration. The need to inject high doses was associated with adverse effects and poor outcomes, as the large amount of Gd around the tumor shielded it from neutron irradiation.
This outcome implies that SMDC can effectively transport suitable amounts of Gd complexes into tumor tissues.”
The nanoscale supports the selective accumulation of therapeutic concentrations and optimal distribution of the drug within the tumor. Smaller molecules can leave the blood capillaries and, as a result, produce higher anti-tumor activity.
Given that the diameter of SMDC is less than 10 nm, our findings likely arise from the profound tumor penetration of SMDC, facilitating the evasion of the shielding effect of thermal neutrons and ensuring efficient diffusion of electrons and γ-rays post thermal neutron exposure.”
What are the implications?
SMDC-Gd could potentially induce high relaxivity with a small Gd payload.”
Single polymer strands can affect the kidneys quite differently based on their chemistry and form, such as dense self-folding like SMDC-Gd4 or flexible polymer like PEGA-Gd4. This could support the development of optimized SMDCs for better tumor diagnosis, even when more than one MRI injection is required.
Our findings underscore the potential of fine-tuning NCAs through self-folding molecular design, marking a significant advancement for NCA utilization in cancer diagnosis and treatment.”
Journal reference:
- Gao, S., Miura, Y., Sumiyoshi, A., et al. (2023). Self-folding macromolecular drug carrier for cancer imaging and therapy. Advanced Science. doi:10.1002/advs.202304171.