Sepsis is a serious condition that occurs when the body reacts to infection improperly. According to a recent estimation, around 48.9 million people across the world develop sepsis, and many die every year from this condition. Early detection of sepsis is critical because it drives the overall disease outcome.
Several strategies, such as fluid resuscitation and antibiotic administration, have been employed to treat sepsis in hospitals. It is difficult to identify people with sepsis because of the heterogeneity linked with this condition. To overcome this challenge, predictive models have been designed for early detection of sepsis.
A deep-learning model can be significantly useful in predicting sepsis because it has the capacity to analyze nonlinear, temporal, and complex correlations among risk factors. In addition, this model can tackle large, multimodal datasets containing clinical notes, radiology imaging, and wearable sensor data. Another advantage of deep learning-based models is their flexible framework for transfer learning.
As mentioned before, COMPOSER is a deep learning-based predictive model for sepsis, which obtains real-time health data from electronic records to predict the condition. A limited number of sepsis algorithms have been tested to understand their effectiveness with regard to patient outcomes. It must be noted that the existing algorithms have exhibited poor positive predictive value (PPV). A frequent false positive alert of these models has developed significant mistrust over their effectiveness. In contrast to these algorithms, COMPOSER was designed to reduce false alarms by identifying odd samples.
About the Study
The current study examined the effectiveness of the COMPOSER deep learning model in the early detection of sepsis and its impact on patient outcomes. For risk score analysis, this neural network model incorporated patient demographics, laboratory reports, vital signs, comorbidities, and concomitant medications.
Based on this score, the susceptibility of patients to develop sepsis could be predicted within four hours. Importantly, this model is designed to avoid out-of-distribution samples that could occur due to errors in data entry or unknown cases.
A team of physicians assessed COMPOSER outputs to determine the accuracy and usefulness of the sepsis alerts. Based on the physicians' recommendations, the algorithm was adjusted. During the implementation phase, the nursing staff was provided with appropriate information about the COMPOSER model.
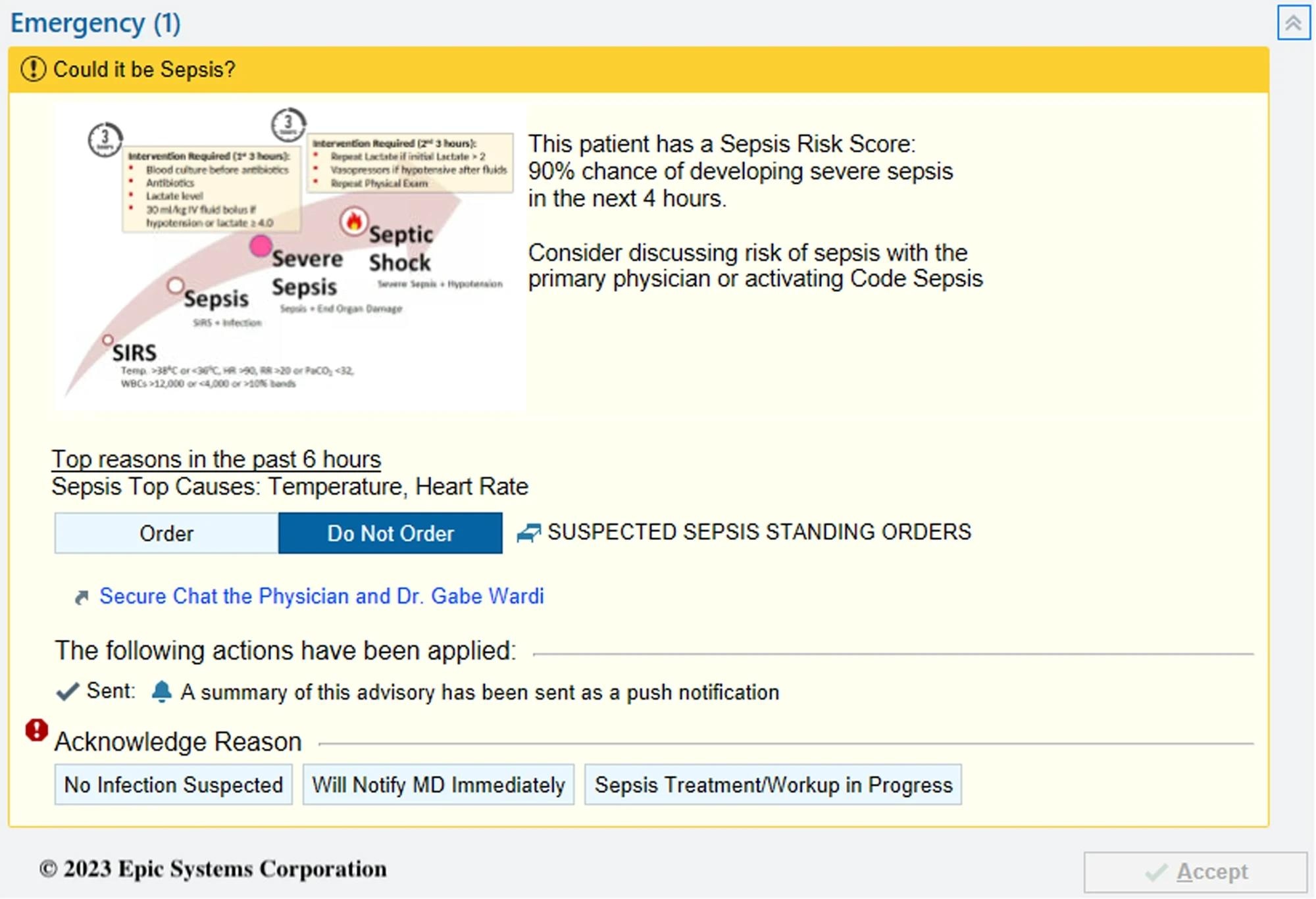 COMPOSER Best Practice Advisory.
COMPOSER Best Practice Advisory.
Study Findings
The present before-and-after quasi-experimental study assessed the effectiveness of a real-time deep-learning model in predicting sepsis in two emergency departments (EDs). Interestingly, a 5.0% increase in sepsis bundle compliance was observed, along with a decrease in in-hospital sepsis-related mortality by 1.9%.
A total of 6,217 patients in EDs between January 1st, 2021, and April 30th, 2023, fulfilled the eligibility criteria of sepsis described in the Sepsis-3 consensus. A total of 5065 patients were identified in the pre-intervention phase and1152 in the post-intervention phase.
The majority of patients with sepsis exhibited similar levels of comorbidity. Similar baseline characteristics were observed in pre-intervention and post-intervention cases. During the post-intervention period, approximately 235 alerts were generated per month by nursing staff. It was estimated that each nurse generated around 1.65 alerts every month.
The current study demonstrated that the use of deep learning models in clinical settings would considerably improve intermediate sepsis outcomes, such as less organ injury at 72 hours from sepsis onset. Based on the model prediction, 55% of patients received antibiotics sooner, which could have contributed to the reduction in in-hospital mortality.
It must be noted that the COMPOSER deep-learning model was able to reduce false alarms significantly. This significantly reduced unnecessary time spent on false diagnoses and resources used for the same.
Conclusions
The current study has some limitations, including a lack of randomization. This inhibited the determination of causal inferences or provided mechanistic insights into them. Although a large cohort from two EDs was considered in this study, external validation from other hospitals is also needed. The impact of the alerts generated by the deep learning sepsis model on patients, particularly those who did not ultimately develop sepsis, was not assessed.
Despite the limitations, this study demonstrated that the use of deep-leaning-based sepsis prediction models in clinical settings could positively reduce in-house mortality. For instance, around 55% of cases were transferred to physicians as soon as possible, who received timely antibiotic intervention. This resulted in a decrease in organ injury at 72 hours. Furthermore, this model also enabled greater sepsis bundle compliance.