This study furthers progress in understanding the Ku70-mediated Ras-ERK signaling pathway and Ku70 activation's molecular mechanisms. These, in turn, could form the basis for the future development of DNA-based therapeutics.
 Study: Ku70 senses cytosolic DNA and assembles a tumor-suppressive signalosome
Study: Ku70 senses cytosolic DNA and assembles a tumor-suppressive signalosome
What is Ku70?
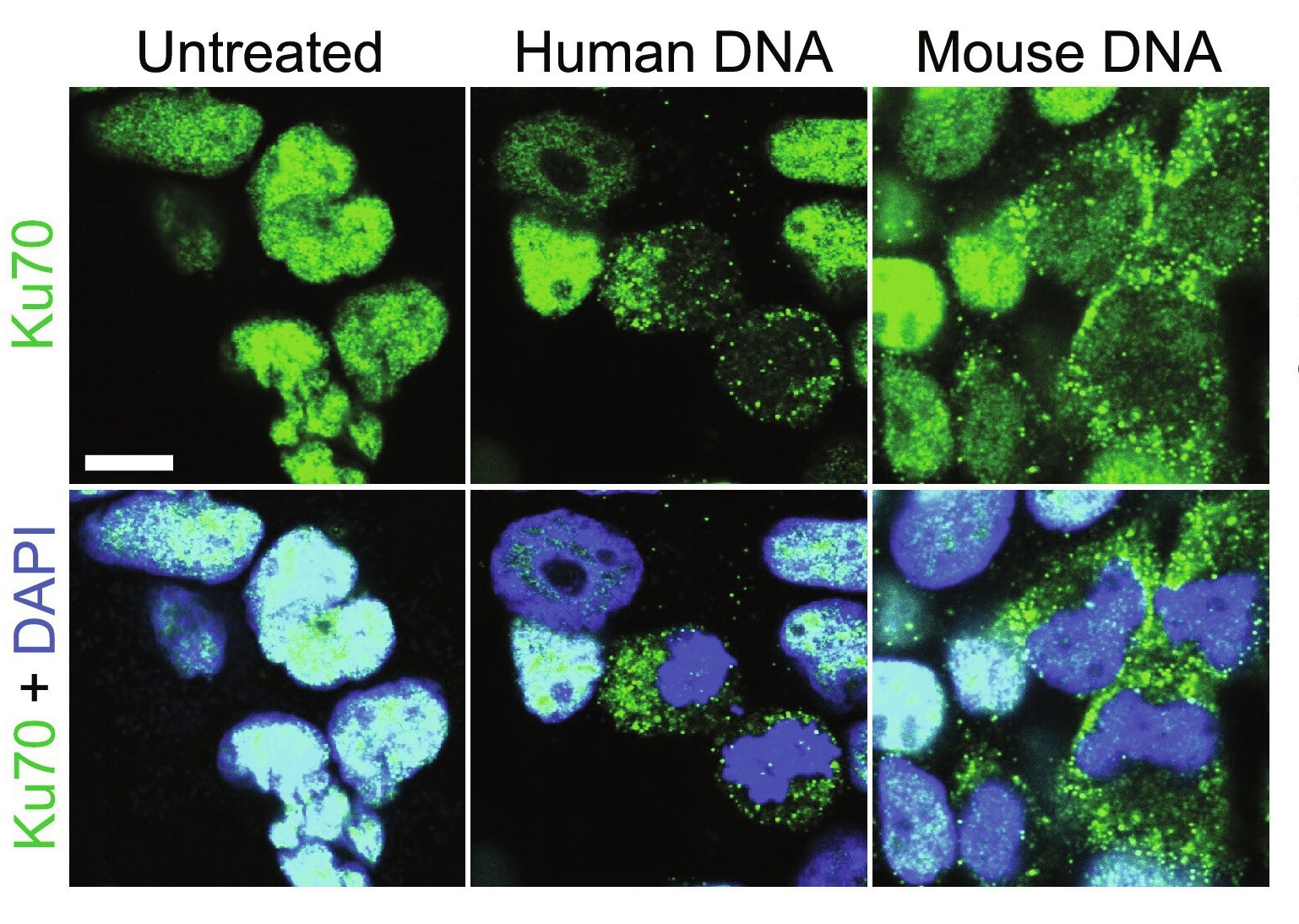
Ku70 is a DNA repair subunit protein that helps repair DNA via the non-homologous end-joining (NHEJ) pathway. In humans, the protein is encoded by the XRCC6 gene, which studies have revealed is evolutionarily conserved. Ku70 can be found both in the cell nucleus and cytoplasm and, until recently, was thought to be restricted to its primary (DNA repair) role. Over the last decade, however, a growing body of literature suggests secondary protein functions, including antimicrobial and anti-tumor.
Cytoplasmic Ku70 has been shown to produce type III interferons (IFNs) in response to bacterial or viral DNA in both human and murine model systems. It has additionally been shown to bind to Rickettsia conorii, a human bacterial pathogen, thereby facilitating its neutralization by nonphagocytic mammalian cells.
Recently, research has suggested that the protein may also have a tumor-suppressive role. In an unrelated experiment, genetically altered mice lacking the Ku70 gene were found remarkably susceptible to hepatocellular carcinoma and spontaneous T-cell lymphoma development. Another study revealed genetic deletion of Ku70 to enhance colorectal cancer risk. Unfortunately, the Ku70 gene is implicated in growth, with its deletion resulting in stunted murine growth. Since smaller body size and poor growth would have confounded interpretation of these results, the association between Ku70 and cancer remains speculative and hitherto unconfirmed.
About the study
The present study aims to elucidate any association between Ku70 protein expression and intestinal cancer risk. Once the association is identified, the mechanism underlining Ku70's protective anti-tumor function is explored. The experimental sample group consisted of wild-type (WT), Ku70+/−(heterozygous for Ku70), Ku70−/−(homozygous recessive), and C57BL/6NcrlAnu transgenic mice. All four mice types were equally divided into case (AOM-DSS) and control (untreated) cohorts.
The study began with the experimental induction of colitis and colitis-associated colorectal tumorigenesis in the case-cohort via the intraperitoneal injection of 10 mg of azoxymethane (AOM). This was followed five days later by administering 1.5% Dextran Sodium Sulfate (DSS) for six days. Fourteen days after AOM administration, mice were euthanized, and their intestines and colon tissues were harvested for methodological validation and downstream analysis.
Induced cancers were identified and characterized using histology, immunohistochemistry, and microscopy techniques. Ku70 and related proteins (e.g., cytokines) were identified and quantified using immunoblotting and enzyme-linked immunosorbent assays (ELISAs), respectively. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) was used to isolate, amplify, and identify RNA of interest within the colon tissue. RNA was further purified using lithium chloride (LiCl) precipitation. Genomic DNA obtained from mouse feces was used to identify and characterize gut microbiome assemblages using 16S ribosomal RNA (rRNA) gene sequencing.
Separately, cell line-based lines of evidence were obtained by constructing a plasmid containing the Ku70 gene, which was then transformed into NEB 5-alpha competent Escherichia coli. Finally, the recombinant E. coli was used to transfect the study subjects, namely HEK293T human cell lines and colon cells cultured from harvested colon tissue.
Cell line analyses incorporated genomics, immunoblotting, and immunofluorescence techniques used for the murine models and included coimmunoprecipitation, proliferation, and organoid culture analyses. Active RAS was screened as a confounding variable using the Active Ras Detection Kit. RAS is a gene family whose mutations are estimated to account for 95% of pancreatic and 45% of colorectal cancers.
Study findings
This study's highlight is validating that the cytosolic DNA sensor Ku70 has the secondary role of tumor suppression. Reductions in Ku70 expression or mutation in its gene were rapidly followed by tumorigenesis in both murine models and cell cultures. This study further unravels the mechanism of action of Ku70, which depicts an unexpectedly high mutation co-occurrence with genes encoding ARAF, RAF1, HRAS, NRAS, and BRAF, RAS family genes previously implicated in intestinal cancers.
"Our study suggests that the Ku70-ERK signaling pathway is tumor suppressive, which is in contrast to the observation that Ras/Raf mutations, which are common in colorectal cancer, drive aberrant activation of downstream ERK-MAPK signaling."
Study findings further suggest that Ku70 may function in a cell-specific manner – epithelial and stromal cells from patients with Crohn's disease or colorectal cancer display decreased Ku70 gene expression even in homozygous dominant conditions. Parallelly, Ku70 was found to form a cytosolic signalosome consisting of Ras, Raf, and Ku70, which docks at the endosome's membrane and mediates the MEK-ERK-Cdc25A-CDK1 signaling axis activation, thereby resulting in an antitumorigenic effect.
"…activation of the Ras-ERK pathway protects mice against colitis (83) and inhibits mammalian cell proliferation. Further studies are required to elucidate which cell types undergo Ras-ERK signaling for the progression of colorectal cancer and which cell types undergo Ku70 signaling for the attenuating of colorectal cancer."
"We speculate that the activation of the Ku70-mediated Ras-ERK signaling might be initiated by the cytoplasmic DNA arising from the gut microbiome introduced into the host cells following a rupture of the intestinal barrier. However, it is also possible that damaged nucleus and/or mitochondria may be a source of cytoplasmic DNA that triggers Ku70-mediated Ras-ERK signaling."
Journal reference:
- Pandey, A., Shen, C., Feng, S., Tuipulotu, D. E., Ngo, C., Liu, C., Kurera, M., Mathur, A., Venkataraman, S., Zhang, J., Talaulikar, D., Song, R., Wong, L., Teoh, N., Kaakoush, N. O., & Man, S. M. (2024). Ku70 senses cytosolic DNA and assembles a tumor-suppressive signalosome. Science Advances, DOI – 10.1126/sciadv.adh3409, https://www.science.org/doi/10.1126/sciadv.adh3409