Obesity is a chronic disease affecting about 650 million individuals worldwide. It increases the risk of several metabolic complications, including cardiovascular disease and type 2 diabetes (T2D). Lifestyle interventions form the core of obesity management. Still, the average weight loss (WL) is ≤ 10%, even with the most intensive interventions.
Moreover, weight maintenance is challenging as weight regain occurs over time. While 5% to 10% WL is clinically beneficial, higher WL is required to improve or attain remission of obesity complications. Furthermore, bariatric surgery can offer up to 30% WL and longer-term weight maintenance; nevertheless, people may not opt for bariatric surgery due to perceived postoperative risks.
Understanding the role of entero-pancreatic hormones resulted in the development of glucagon-like peptide (GLP)-1 receptor agonists (RAs) for obesity and T2D treatment. Semaglutide is the latest GLP-1 RA approved for obesity, which can result in 15% to 17% mean WL. Nonetheless, a significant difference exists between WL achieved through obesity pharmacotherapies and bariatric surgery.
Further, current GLP-1 RAs for obesity are injectables, which some people may not consider. However, oral RAs are being developed to increase acceptance and adherence. Besides, a pipeline of pharmacotherapies based on entero-pancreatic hormones is being developed to enhance or complement GLP-1 RAs. In the present study, the authors discussed the pipeline of obesity pharmacotherapies.
 Study: What is the pipeline for future medications for obesity? Image Credit: Suzanne Tucker / Shutterstock
Study: What is the pipeline for future medications for obesity? Image Credit: Suzanne Tucker / Shutterstock
GLP-1 RAs
Subcutaneous semaglutide (2.4 mg) and liraglutide (3 mg) have been approved for obesity management. A higher semaglutide dose (7.2 mg) is being evaluated in a phase 3 trial. Because people may be reluctant to use injectables, oral semaglutide has been introduced and approved for T2D, with the 14 mg dose improving hemoglobin A1c (HbA1c) levels and WL.
A phase 3 trial evaluated the efficacy and safety of a 50 mg oral dose of semaglutide in non-T2D obese subjects over 68 weeks. The trial revealed that semaglutide recipients achieved over 17% WL while placebo subjects attained < 2% WL. In a different trial on T2D subjects, the 50 mg semaglutide dose resulted in approximately 10% WL compared to 5.4% WL with the 14 mg dose.
Danuglipron is a non-peptide, G protein-based, oral GLP-1 RA. A phase 2b investigation among obese subjects showed that 40 mg to 200 mg doses of danuglipron resulted in over 11% WL after 32 weeks. Orforglipron is another non-peptide, oral GLP-1 RA, which is being evaluated for T2D and obesity management.
In obese subjects, 36-week orforglipron treatment led to WL of ≤ 14.7% and improvements in cardiometabolic risk factors. Likewise, in T2D patients, nearly half of the participants attained 10% or higher WL after a 26-week orforglipron treatment in a phase 2 trial. Currently, several phase 3 trials on orforglipron and oral semaglutide are underway for different populations.
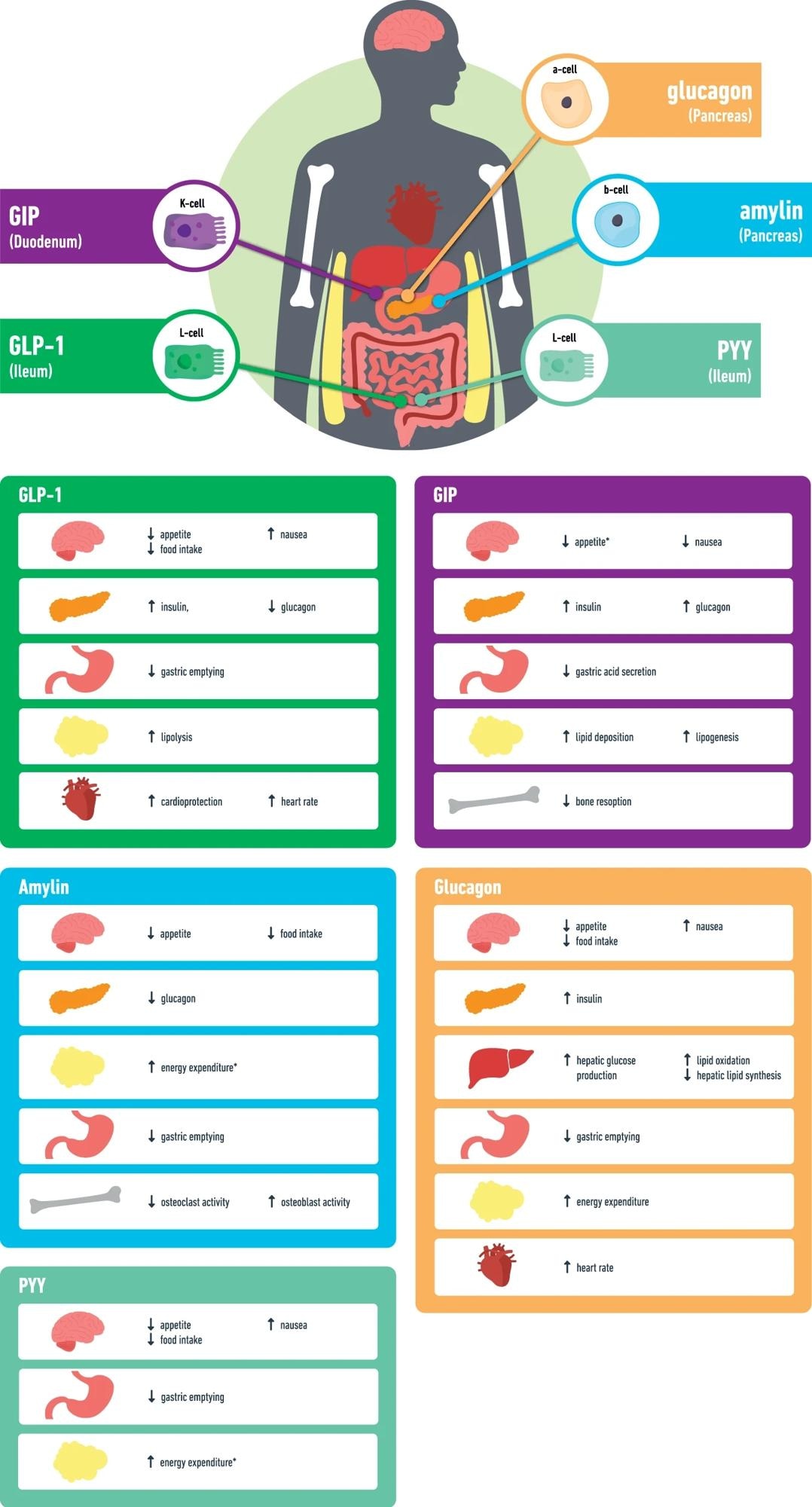
GLP-1 glucagon like peptide-1, GIP glucose-dependent insulinotropic polypeptide, PYY peptide YY, *data mainly from animal studies.
Entero-pancreatic hormones and their combinations for obesity management
Multiple entero-pancreatic hormones are currently evaluated alone or in combination with GLP-1 RAs to augment or complement the effects of GLP-1 agonism on weight and metabolism. Jejunal K-cells secrete glucose-dependent insulinotropic peptide (GIP) in response to food intake. GIP stimulates insulin release and increases lipogenesis, glucagon secretion, and lipid buffering capacity.
Animal studies suggest an anorexigenic action of GIP receptor agonism. A phase 1 trial recently showed that repeated doses of long-acting GIP RA caused a modest WL in T2D subjects. Tirzepatide is an unimolecular subcutaneous dual (GIP and GLP-1) RA with comparable affinity to the GIP receptor but lower GLP-1 receptor affinity.
A phase 3 study investigated the efficacy and safety of tirzepatide for obesity, and it has now been approved for chronic weight management. Various trials are evaluating the effectiveness and safety of tirzepatide in ameliorating cardiometabolic complications. Further, several injectable or oral GIP/GLP-1 RAs are in the early phases of development.
Preliminary findings from rodent studies indicated a synergistic role of dual glucagon and GLP-1 agonism in reducing food intake. As such, numerous glucagon/GLP-1 co-agonists have been developed. Notwithstanding the promising results of glucagon/GLP-1 co-agonism in experimental studies, the efficacy and tolerability of the co-agonists have been heterogeneous in obese subjects.
A triple agonist targeting glucagon, GIP, and GLP-1 could result in superior WL and glycemic control than dual agonists. For instance, retatrutide is a triple agonist and has been shown to improve WL and glucose profile in preclinical models relative to tirzepatide through reduced calorie intake and elevated energy expenditure. A phase 2 study in non-T2D obese individuals reported dose-dependent WL following 48-week treatment with varying doses of retatrutide.
Concluding remarks
Taken together, a new era has commenced for obesity treatment where combinations of entero-pancreatic hormones can reach the WL efficacy of bariatric surgery. Besides tirzepatide, the dual agonist approved for chronic management, various dual and triple agonists are evaluated in phase 3 trials.
The multitude of obesity treatment options will enable tailored therapies based on individuals’ preferences, treatment responses, and comorbidities. Overall, obesity pharmacotherapies represent a rapidly growing field, and research on efficacy, safety, and cost-effectiveness will inform their place in therapeutic options for obesity and related complications.