In a recent study published in the journal Cell, a team of scientists predominantly from Stanford University discovered that sex-biased autoimmunity, where females are more affected by autoimmune disorders than males, is primarily driven by the Xist ribonucleoprotein complex containing various autoantigenic components.
 Study: Xist ribonucleoproteins promote female sex-biased autoimmunity. Image Credit: Kateryna Kon / Shutterstock
Study: Xist ribonucleoproteins promote female sex-biased autoimmunity. Image Credit: Kateryna Kon / Shutterstock
Background
After cancer and cardiovascular disease, autoimmune disorders are the most prevalent category of disease, with females having a four-fold higher incidence of autoimmune diseases than males. Sjögren’s disease has a 19 to one female to male prevalence, while the sex ratio of systemic lupus erythematosus patients is 9:1 for females to males. Furthermore, Klinefelter syndrome patients who have XXY sex chromosomes and are phenotypically male with hormonal patterns of a biological male also have the same risk of autoimmune disorders as females.
While the role of hormones has been extensively studied in relation to autoimmune disorders, research indicates that irrespective of hormone status and sex, X chromosome dosage seems to be one of the major drivers of autoimmune disease risk. Additionally, studies among identical twins indicate that the penetrance of autoimmune disease can also vary, suggesting that environmental factors can influence the genetic disposition to autoimmune disorders. X-linked genes such as toll-like receptor 7 (TLR7) have been thought to contribute to the development of some autoimmune disorders.
About the study
In the present study, the researchers used autoimmune-resistant and autoimmune-prone mouse models, C57BL/6J and SJL/J, respectively, to understand the role of X chromosome dosage compensation in determining the disproportionate risk of autoimmune diseases in females.
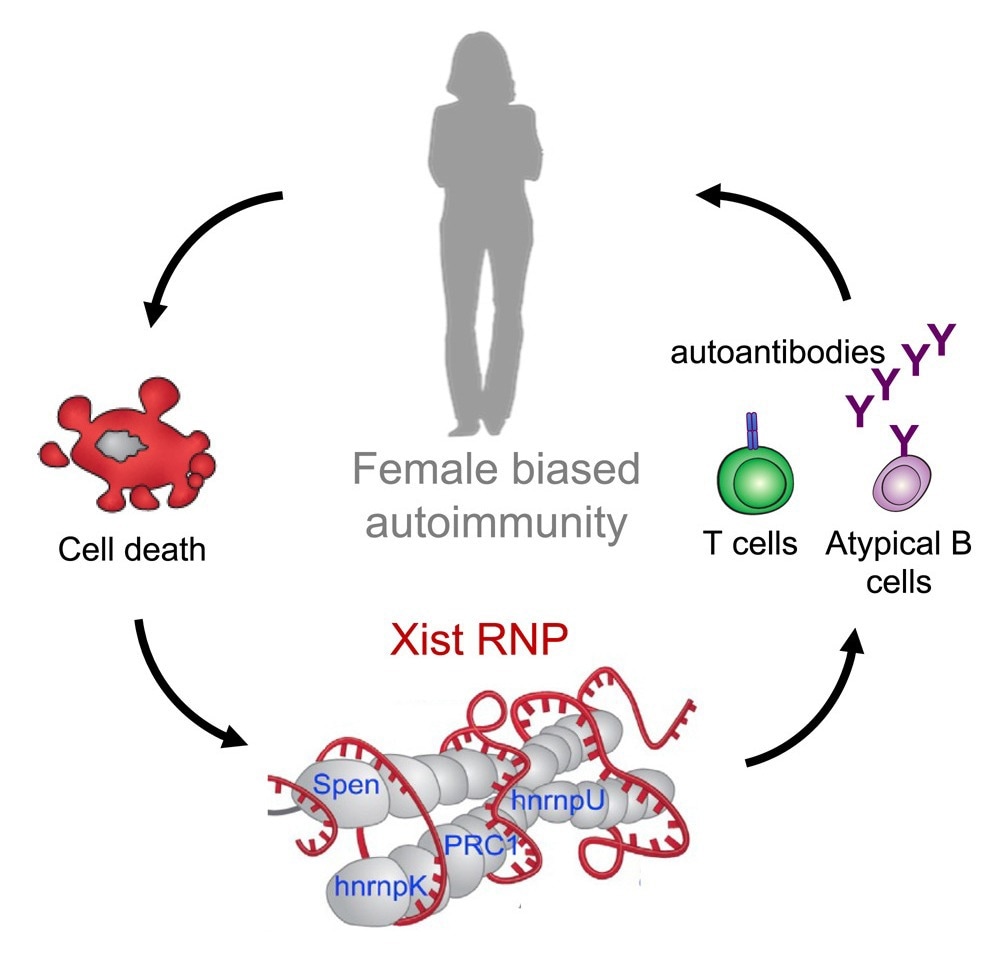
Since mammalian females have two copies of the X chromosomes as compared to mammalian males, who have an XY genotype, one of the two X chromosomes in females is epigenetically silenced for dosage compensation in every cell through a mechanism involving a 19-kb pair long non-coding ribonucleic acid (lncRNA) called Xist. Xist is not expressed in males, and only the inactive X chromosome transcribes this lncRNA in females.
Studies in embryonic stem cells from mice have shown that X chromosome inactivation is established when Xist forms a ribonucleoprotein complex with 81 binding proteins that are unique to this complex. Xist binds directly to 10 of these binding proteins through RNA-protein interactions, and to the remaining 71 indirectly through protein-protein interactions. Several of these binding proteins have previously been identified as autoantigens and are thought to activate innate immune system pathways through toll-like receptors.
Here, the researchers used non-silencing alleles of Xist that were inducible and introduced them into the autosomes of the autoimmune-resistant and autoimmune-prone mice strains. The induction of Xist ribonucleoprotein complex formation in male mice of a chemically induced systemic lupus erythematosus mouse model allowed this female-specific process to be observed in a male background.
RNA sequencing and ATAC-sequencing or assay of transposase-accessible chromatin by sequencing were used to assess changes in gene expression in splenic CD4+ T cells and potential transcription regulation alterations. Principal component analysis was also used to determine similarities between male mice expressing the induced Xist allele.
Serum samples from treated mice were also assessed for antigens against scleroderma and systemic lupus erythematosus. Additionally, serum samples obtained from human patients of systemic lupus erythematosus, dermatomyositis, and scleroderma were tested for reactivity against proteins from the Xist ribonucleoprotein complex.
Results
The results reported that the induction of transgenic expression of non-silenced Xist in male mice formed Xist ribonucleoprotein complexes and led to the production of autoantibodies. Male autoimmune-prone mice models of pristane-induced systemic lupus erythematosus showed multi-organ pathology that was more severe than that seen in the wild-type mice. Furthermore, the expression of Xist in the male mice had reprogrammed the chromatic states and the B and T cell populations to be more similar to those found in wild-type female mice.
The reactivity against multiple proteins from the Xist ribonucleoprotein complex was also found to be significant in the serum samples obtained from human systemic lupus erythematosus, dermatomyositis, and scleroderma patients.
The findings highlight the potential for using these Xist ribonucleoprotein complex-associated proteins as novel antigens for detecting and monitoring autoimmune diseases. The discovery of atypical B cell accumulation due to Xist ribonucleoprotein complex expression also provides a potential area of research for autoimmune disorder therapy.
Conclusions
Overall, the findings indicated that the Xist ribonucleoprotein complex selectively expressed in females and involved in X chromosome dosage compensation drives sex-biased autoimmunity. Patients with autoimmune conditions such as scleroderma and systemic lupus erythematosus have higher reactivity against proteins from the Xist ribonucleoprotein complex, highlighting the potential use of these proteins as antigens for screening and early detection of autoimmune disorders.
Journal reference:
- Dou, D. R., Zhao, Y., Belk, J. A., Zhao, Y., Casey, K. M., Chen, D. C., Li, R., Yu, B., Srinivasan, S., Abe, B. T., Kraft, K., Hellström, C., Sjöberg, R., Chang, S., Feng, A., Goldman, D. W., Shah, A. A., Petri, M., Chung, L. S., & Fiorentino, D. F. (2024). Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell, 187(3), 733-749.e16, 10.1016/j.cell.2023.12.037, https://www.cell.com/cell/fulltext/S0092-8674(24)00002-3