In this insightful interview from SLAS 2024, we talk with Dr. Abidemi Junaid, a scientist at the Hansjörg Wyss Institute for Biologically Inspired Engineering at Harvard University. Junaid spearheads the development of the human Vagina Chip, a pioneering tool designed to study host-microbiome interactions in bacterial vaginosis and pave the way for biotherapeutic development and validation.
With a rich background in biometrics, systems biomedicine, and pharmacology, Junaid's interdisciplinary approach has culminated in the creation of a model that closely replicates the human vaginal environment. Here, Junaid shares insights into the challenges and triumphs of simulating the female reproductive system on a chip, the implications for women's health research, and the future of biotherapeutic strategies beyond bacterial vaginosis.
Firstly, please introduce yourself and outline your career to date. More specifically, please provide us with an outline of the research you are presenting on a human Vagina Chip to study host-microbiome interactions in bacterial vaginosis for biotherapeutic development and validation here at SLAS.
My name is Abidemi Junaid, and I am a scientist at Hansjörg Wyss Institute for Biologically Inspired Engineering at Harvard University. I lead the overall effort on the advancement of preclinical testing and modeling of the human reproductive tract using organ-on-chip technology.
The reproductive health of a woman is strongly associated with a vaginal microbiome mainly composed of Lactobacillus species. In contrast, dysbiosis decreases this population and increases the diversity of anaerobic species, including pathogens, such as Garderenella vaginalis, as seen in bacterial vaginosis (BV).
BV increases the risk of pre-term birth, miscarriages, and the chances of acquiring sexually transmitted diseases. Various therapeutic strategies are being explored to modulate the composition of the vaginal microbiome; however, there is no human-relevant preclinical model that faithfully reproduces the vaginal epithelial microenvironment for validation of potential therapeutics.
At SLAS, I will describe our human Vagina Chip that is lined by hormone-sensitive, primary vaginal epithelium interfaced with underlying stromal fibroblasts, which sustains a low physiological oxygen concentration in the epithelial lumen.
The Vagina Chip allows us to study a human model of the vaginal microbiome and develop new treatments for BV and other conditions that threaten women’s health.
Firstly, for our readers, please could you tell us more about what organ-on-a-chip technologies are, and more specifically about the benefits of the human vagina chip compared to that of an animal model?
Organs-on-chips (OoCs) are systems containing engineered or natural miniature tissues grown inside microfluidic chips. To better mimic human physiology, the chips are designed to control cell microenvironments and maintain tissue-specific functions.
Animal models are of limited use in research to study host-microbiota interactions in the vaginal space because of the major physiological, anatomical, and microbial differences present in these models compared to the human vagina. The Vagina Chip replicates the human vaginal tissue microenvironment including its microbiome in vitro.
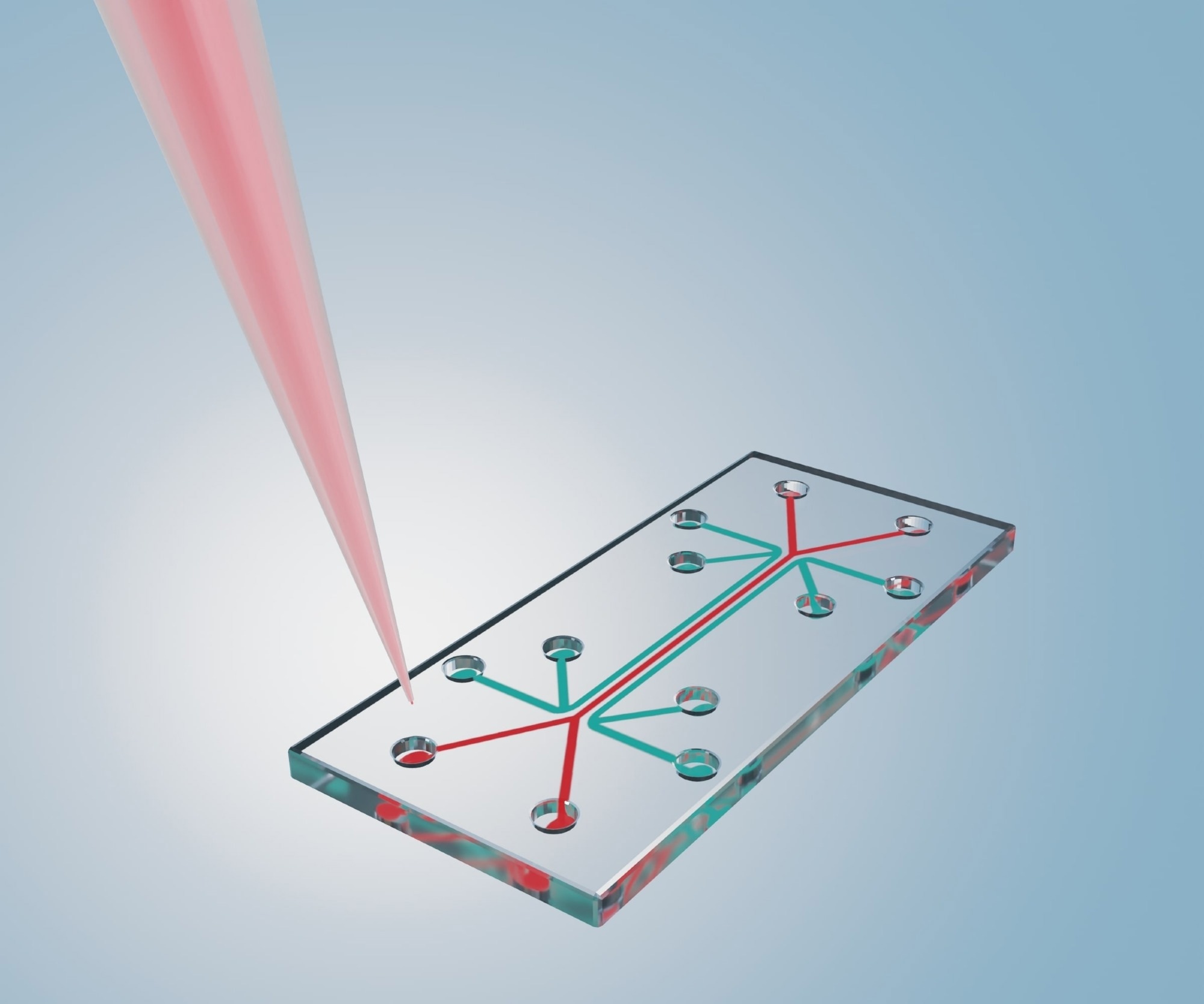 Image Credit: Love Employee/Shutterstock.com
Image Credit: Love Employee/Shutterstock.com
How does the vagina chip technology simulate the unique environment of the female reproductive system, and what are its applications in women's health research?
The Vagina Chip supports the growth of healthy microbiome community on-chip, which is accompanied by maintenance of epithelial cell viability, accumulation of D- and L-lactic acid, maintenance of a physiologically relevant low pH, and down regulation of proinflammatory cytokines.
The Vagina Chip can be used to better understand interactions between the vaginal microbiome and host tissues, as well as to evaluate the safety and efficacy of live biotherapeutics products.
What is organ-on-a-chip technology?
Your journey from biometrics to systems biomedicine and pharmacology is quite impressive. Could you share how your diverse academic background has influenced your approach to your current research on the human Vagina Chip?
My interdisciplinary background has allowed me to apply various elements from engineering, chemistry, and biology in the further development of the human Vagina Chip to recapitulate the human vagina for biotherapeutic studies successfully.
During my Ph.D., I learned how to use the high-throughput human microvessels-on-chips for screening patient samples and drug discovery. I was able to use these skills for studying microbes isolated from vaginal clinical swab samples in the Vagina Chip to mimic healthy and dysbiotic conditions.
The Vagina Chip presents a novel approach to studying bacterial vaginosis (BV). Can you describe the initial challenges you faced in replicating the complex vaginal microenvironment on a chip?
One of the initial challenges was getting the cells that we cultured in the Vagina Chip to differentiate and become stratified just like the human vagina. We were able to solve that by using homemade differentiation media and physiologically relevant dynamic flow of media in the system.
Learn more about vaginal dysbiosis
The study indicates that Lactobacillus-rich live biotherapeutic products (LBPs) can alleviate dysbiotic responses without eradicating G. vaginalis. How does this finding challenge or support existing theories about BV treatment?
In a healthy vaginal microbiota, you a have high population of L. crispatus and very low population of G. vaginalis. Since we still see a high population of G. vaginalis after treatment with the LBP in our Vagina Chip, this indicates that additional treatment is needed to reduce the population of G. vaginalis and finally reach a healthy state.
The Vagina Chip's ability to correlate pro-inflammatory responses with untreated BV patient samples is intriguing. How do you envision this capability impacting the future of personalized medicine for reproductive health?
There is growing recognition that taking care of women’s health is critical for the health of all humans, but the creation of tools to study human female physiology is lagging.
We’re hopeful that this new preclinical model will drive the development of new treatments for BV as well as new insight into female reproductive health. Furthermore, this model will allow us to study individual patients from different ethnicities and develop therapies that is specific to each of them.
You mention that further reduction in G. vaginalis numbers might produce a greater therapeutic effect. What strategies or modifications to the Vagina Chip are being considered to investigate this hypothesis?
One of the strategies that we are trying is to treat the dysbiotic Vagina Chip with hydroxy-metronidazole and the LBP. Hydroxy-Metronidazole is an antibiotic that is commonly used to kill G.vaginalis in BV patients.
However, treating with hydroxy-metronidazole alone can lead to recurrent BV. We hope that with the combination of hydroxy-metronidazole and LBP there is a lower chance of recurrence.
Given the complexity of the vaginal microbiome and its impact on women's health, how do you see your work influencing the development and validation of other biotherapeutic strategies beyond BV?
Various therapeutic strategies are being explored to modulate the composition of the vaginal microbiome; however, there is no human model that faithfully reproduces the vaginal epithelial microenvironment for preclinical validation of potential therapeutics or testing hypotheses about vaginal epithelium-microbiome interactions.
The Vagina Chip is a preclinical model of the human vaginal mucosa that can be used to understand better interactions between the vaginal microbiome and host tissues, as well as to evaluate the safety and efficacy of live biotherapeutics products. This will help us to predict how successful a biotherapeutic strategy would be in clinical trials.
Finally, as someone at the forefront of organ-on-chip technology, what advice would you give to young scientists interested in entering this field, and what do you think are the most exciting possibilities on the horizon?
The field of organs-on-chips is very interdisciplinary. So, I advise young scientists to explore research in various scientific areas and collaborate with people with different scientific backgrounds.
I look forward to the time when we can fully use organ-on-a-chip technology to replace animal models. Moreover, this technology will be an important tool to decide whether a therapy should go to clinical trial.
Where can readers find more information?
About Abidemi Junaid, Ph.D.
 Abidemi Junaid received his B.S. in Biometrics from Zuyd University of Applied Sciences, M.S. in Biomolecular Sciences from VU University, and Ph.D. in Systems Biomedicine and Pharmacology from Leiden University. His Ph.D. work was centered around the development of high-throughput human microvessels-on-chips for studying microvascular destabilization, infectious diseases, and metabolomics.
Abidemi Junaid received his B.S. in Biometrics from Zuyd University of Applied Sciences, M.S. in Biomolecular Sciences from VU University, and Ph.D. in Systems Biomedicine and Pharmacology from Leiden University. His Ph.D. work was centered around the development of high-throughput human microvessels-on-chips for studying microvascular destabilization, infectious diseases, and metabolomics.
He also worked on integrating mechanical fluid flow and biological and environmental sensing in organs-on-chips. Altogether, this enabled him to identify the impact of patient plasma on microvessels for clinical studies. As a Scientist at the Wyss Institute, he is working on advancing preclinical testing and modeling of the human immune system using organ-on-chip technology.