In a recent study published in the journal Acta Neuropathologica, researchers identified protective genetic variations in APOEε4 carriers against Alzheimer's disease (AD) using whole-genome sequencing (WGS) and pathway analysis.
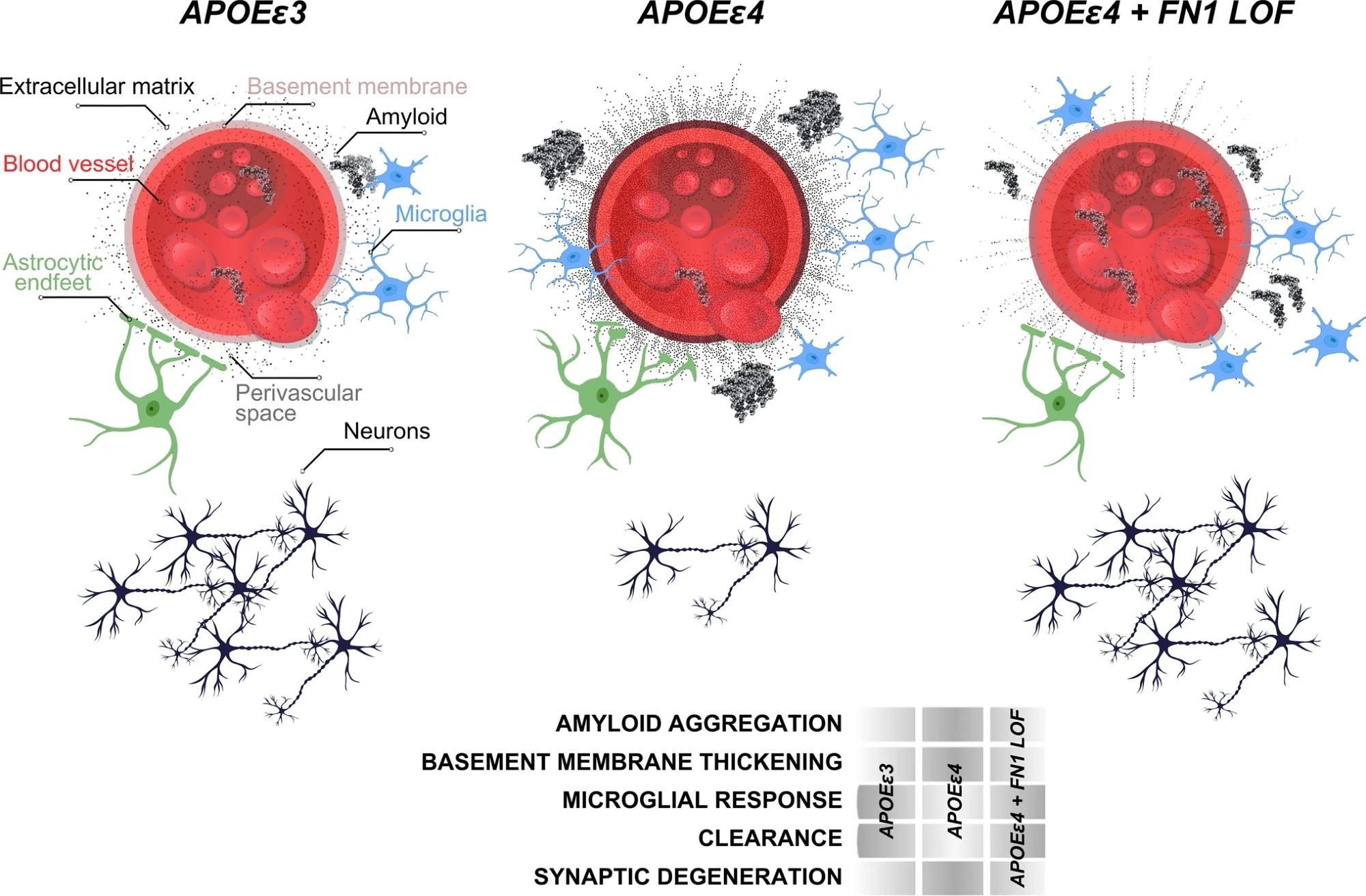 Schematic abstract for the protective effect of FN1 variants. Study: Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease
Schematic abstract for the protective effect of FN1 variants. Study: Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease
Background
AD is characterized by progressive memory loss and cognitive decline, often without symptoms for an extended period. At autopsy, AD brains typically reveal β-amyloid plaques and neurofibrillary tangles of tau protein. Despite advances in understanding, AD remains largely untreatable, with existing drugs providing minimal relief, primarily due to incomplete knowledge of its pathological mechanisms. Genetic factors, notably the APOEε4 allele, significantly influence the disease's variability across individuals and ethnic groups. This allele's effects vary, with some carriers experiencing milder or delayed symptoms, underscoring the need for further research to explain these complex genetic interactions and develop better treatments and diagnostics.
About the study
All human samples in the study were de-identified, ensuring that researchers could not infer or obtain personal information from the donors. Prior to generating clinical data, approvals were secured from the Institutional Review Board at both Columbia University Irving Medical Center and Mayo Clinic. The study also included animal experiments, which adhered to the Institutional Animal Care and Use Committee (IACUC) guidelines at Columbia University under the approved protocol ACAABN3554. This program follows AAALAC International accredited standards and maintains an Animal Welfare Assurance with the Public Health Service.
For the zebrafish studies, both wild-type AB strains and fn1b−/− homozygous knockout fish lines, ranging from 8 to 10 months old and of both genders, were utilized. Animals were randomly assigned to experimental conditions from the same clutch to ensure consistency.
Human cohorts featured in this study included the NIA-AD Family Based Study (NIA-AD FBS), which involved multiplex families across the USA. Families were selected based on the presence of at least one member diagnosed with definite or probable AD post-age 60 and a sibling with a similar onset. Detailed demographic and clinical data were available for these participants. The Washington Heights/Inwood Columbia Aging Project (WHICAP) recruited individuals aged 65 years and older from northern Manhattan to study clinical and genetic risk factors for dementia. The Estudio Familiar de Influencia Genetica en Alzheimer (EFIGA) targeted a different ethnic group, including families from Puerto Rico, the Dominican Republic, and New York, to identify protective alleles in APOEε4 healthy carriers.
All procedures, from the care and use of animals to the handling of S samples, were carried out under ethical standards and protocols approved by relevant authorities, ensuring a high level of scientific integrity and respect for both human and animal subjects involved in the research.
Study results
The present study accessed WGS data from 3,578 individuals across over 700 families of non-Hispanic White and Caribbean Hispanic descent, all affected by AD. Through harmonization and quality control of the WGS data, researchers identified rare coding variants in APOEε4 carriers who were cognitively unaffected and elderly. These carriers, both homozygous and heterozygous, had rare variants not present in non-carriers. The research prioritized variants potentially damaging to protein products, discovering 510 variants in 476 genes, particularly noted in the fibronectin 1 (FN1) gene, among others.
Gene ontology analysis further revealed a strong enrichment for extracellular matrix (ECM)-related processes in the identified variants. This indicated that functional alterations in ECM composition could offer protective mechanisms in APOEε4 carriers, suggesting that APOEε4-related increases in ECM components could be counteracted by loss-of-function variants in these genes. Two significant genes, collagen type VI alpha 2 chain (COL6A2) and FN1, were examined for variants that might influence ECM stability through changes in charged residues, hypothesized to impact protein function.
Further analyses involved an independent cohort from databases such as the Alzheimer's Disease Genetic Consortium (ADGC) and the United Kingdom Biobank (UKB), focusing on non-Hispanic White individuals to test the replication of findings. The variant rs140926439 in FN1 showed a significantly reduced risk of AD in APOEε4 homozygous carriers, corroborating the protective effect of this variant against AD. Additionally, sensitivity analyses confirmed these findings, reinforcing the variant's potential as a protective factor in AD progression.
The study also explored the correlation between APOEε4 dosage and FN1 deposition at the blood-brain barrier. Immunostaining analyses in brain tissues of individuals with different APOE genotypes showed that FN1 levels increased with APOEε4 dosage, particularly in homozygous carriers.
Lastly, the potential role of FN1 in modulating responses to amyloid toxicity was examined using a zebrafish model. The loss of FN1 function in zebrafish led to reduced gliovascular interactions and a decreased gliotic response after amyloid treatment, suggesting that fibronectin could play a crucial role in the regulation of amyloid beta clearance. This aligns with the hypothesis that FN loss-of-function variants could be protective against AD pathology by enhancing the clearance of toxic protein aggregates and modulating immune system activity.