The burden of critical illness is expected to increase with the aging population. Intensive care for critically ill subjects reduces mortality, but delays can lead to adverse outcomes. While most in-hospital arrests could be prevented, identifying the early signs of deterioration could be difficult. Hospital hospitals have deployed rapid response systems (RRSs) to manage clinical decline. The track-and-trigger system (TTS) is critical to RRS activation.
TTS could be integrated into the hospital for real-time alerts, which may improve critical care quality. Previously, the researchers developed an AI-enabled ECG (AI-ECG) to stratify the mortality risk and predict all-cause mortality. They showed that AI-ECG performed better in predicting 30-day mortality than one-year mortality. While AI-ECG can serve as an effective TTS, there are no relevant randomized controlled trials to date.
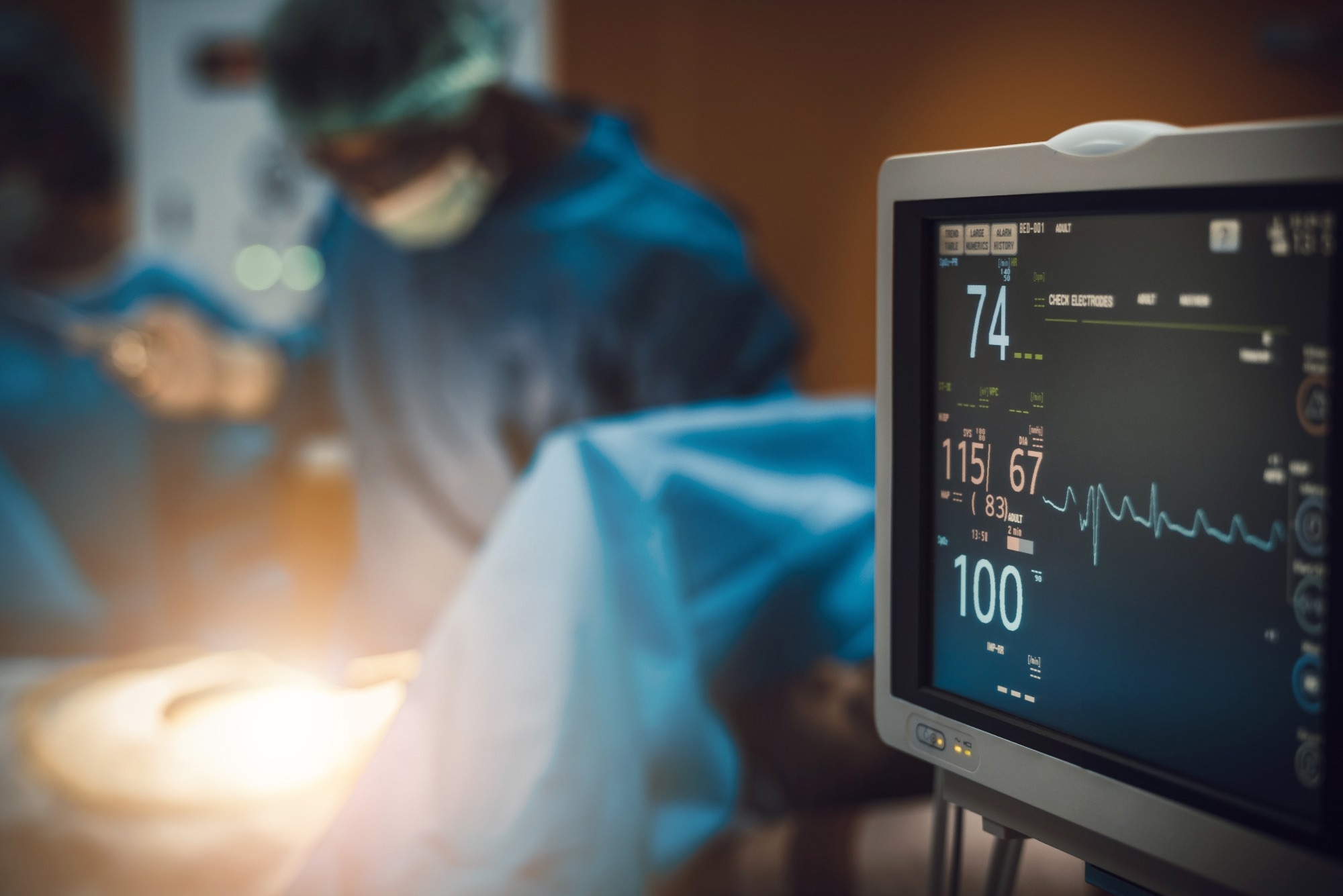 Study: AI-enabled electrocardiography alert intervention and all-cause mortality: a pragmatic randomized clinical trial. Image Credit: totojang1977 / Shutterstock
Study: AI-enabled electrocardiography alert intervention and all-cause mortality: a pragmatic randomized clinical trial. Image Credit: totojang1977 / Shutterstock
About the study
In the present study, researchers applied AI-ECG to TTS to identify deteriorating patients whose conditions may be reversible and evaluate potential benefits. This trial was performed at a community hospital and an academic medical center in Taiwan. Patients’ data were collected from electronic health records (EHRs) and included in the analysis if they received an ECG for any indication between December 15, 2021, and April 30, 2022.
Subjects under 18 years and those with a delay of over two hours between ECG and AI-ECG analysis were excluded. AI-ECG’s output was a value ranging between negative and positive infinity; thus, this was transformed into a percentile score. Patients were categorized as low or high risk based on a prespecified threshold, and the TTS was implemented accordingly.
Several analyses were undertaken to evaluate the performance of the percentile scores. Patients’ characteristics and ECG differences were compared between low- and high-risk groups. Further, the Spearman correlation coefficient analysis was performed using the three most significant variables that correlated with percentile scores. AI-ECG predictions were modeled and ranked using all variables using XGBoost.
The results of machine learning models were compared with AI-ECG scores. Cox proportional hazards models analyzed the relationship between AI-ECG risk stratification and the cause of death. Once AI-ECG indicated a high risk, the physician received an alert message. Physicians were instructed to comprehensively assess patients after receiving the alert and arrange appropriate tests and interventions.
While alerts were specifically sent for high-risk cases, physicians could still access the AI-ECG reports for low-risk patients in the intervention group through electronic health records (EHRs). In contrast, physicians in the control group received the AI-ECG reports without any real-time alerts, following the usual care protocol. The primary endpoint of the study was all-cause mortality within 90 days. Secondary endpoints included detailed cause-of-death analyses, as well as the frequency and types of follow-up tests and medical treatments initiated after the ECG assessments.
Findings
Overall, 39 physicians and 15,965 patients were included. The intervention group included 8,001 patients, while the control group had 7,964 patients. AI-ECG stratified 709 and 688 patients from intervention and control groups as having a high risk of mortality, respectively. Physicians received alerts for patients in the intervention group and accordingly arranged pertinent intensive monitoring or care. The team found that age was highly correlated with AI-ECG risk score.
Further, heart rate and modified early warning score (MEWS) were strongly associated with the score in medium-to-high-risk patients. AI-ECG was significantly better than patients’ baseline characteristics in predicting the mortality risk. The high-risk group had a hazard ratio of 7.53 for all-cause mortality, adjusted for age and sex. Further, its predictive ability was much higher for cardiac deaths compared to non-cardiac deaths.
Notably, the predictive ability was the highest for death due to arrhythmia. There was a significant difference in the cumulative proportion of deaths between groups. The active AI-ECG alerts decreased mortality risk in the intervention group from 23% to 16%. However, an opportunity to review AI-ECG reports for those with a low risk provided only a little help. The high-risk group had a significantly lower risk of cardiac and non-cardiac death.
Conclusions
Taken together, the study demonstrated that AI-ECG use resulted in a significant mortality reduction. The success of this RRS using AI-ECG could be attributed to physicians' higher attention. The team estimates an average of 10 or fewer alerts per month for each patient when deployed in real-time for all patients in the future.
While the precise mechanisms through which the AI-ECG system led to mortality reductions are unknown, two potential explanations have been proposed. First, the system has exceptional risk stratification capabilities, enabling physicians to pay more attention. Second, the system may identify subtle changes in the underlying cardiac conditions from unknown ECG features.