Sleep represents a state of vulnerable inactivity. Given the risks of this vulnerability, it is assumed that sleep may confer some benefit. It has been suggested that sleep clears toxins and metabolites from the brain via the glymphatic system. This notion has essential implications; for instance, diminished toxin clearance due to chronically poor sleep may aggravate Alzheimer's disease.
How toxins and metabolites are cleared from the brain remains unclear, with disputes about the clearance mechanisms and anatomical pathways. According to the glymphatic hypothesis, the bulk fluid flow, driven by hydrostatic pressure gradients from arterial pulsations, actively clears brain parenchyma solutes during non-rapid eye movement sleep. Further, at sedative doses, anesthetics enhance clearance. Whether sleep increases clearance by elevated bulk flow is unknown.
 Brief Communication: Brain clearance is reduced during sleep and anesthesia. Image Credit: vetre / Shutterstock
Brief Communication: Brain clearance is reduced during sleep and anesthesia. Image Credit: vetre / Shutterstock
The study and findings
In the present study, researchers measured fluid movement and clearance in mice's brains. First, the diffusion coefficient of fluorescein isothiocyanate (FITC)-dextran, a fluorescent dye, was determined. FITC-dextran was injected into the caudate putamen, and fluorescence was measured in the frontal cortex.
Initial experiments involved waiting for a steady state, bleaching the dye in a small tissue volume, and determining the diffusion coefficient from the rate of movement of unbleached dye into the bleached zone. The methodology was validated by measuring FITC-dextran diffusion in agarose brain phantom gels that were modified to approximate the brain's optical absorption and light scattering properties.
The distribution of light was approximated by a hemispherical Gaussian distribution. The recovery of fluorescence was recorded 30 seconds after bleaching. The researchers noted that these (recording) data and the theoretically predicted time course were highly concordant. Further, the diffusion coefficient values were concordant with values estimated using a direct method (without photobleaching).
Next, the diffusion coefficient of FITC-dextran was measured in vivo. Once injected into the caudate putamen, it was detectable in the frontal cortex. Fluorescence peaked six to seven hours post-injection and waned at 6% per hour. During the declining phase, the recovery from bleaching was recorded. The fluorescence recovery was consistent with theoretical predictions.
Effective tissue diffusion coefficient values were derived from the time courses, and the vigilance states (sleep, wake, and anesthesia) were determined. The average diffusion coefficient value was 32.1 μm2/s across all vigilance states, corresponding to a tortuosity of -2.5. This was consistent with reported values for rodent neocortex and suggested that FITC-dextran movement within the cortex was mainly due to diffusion.
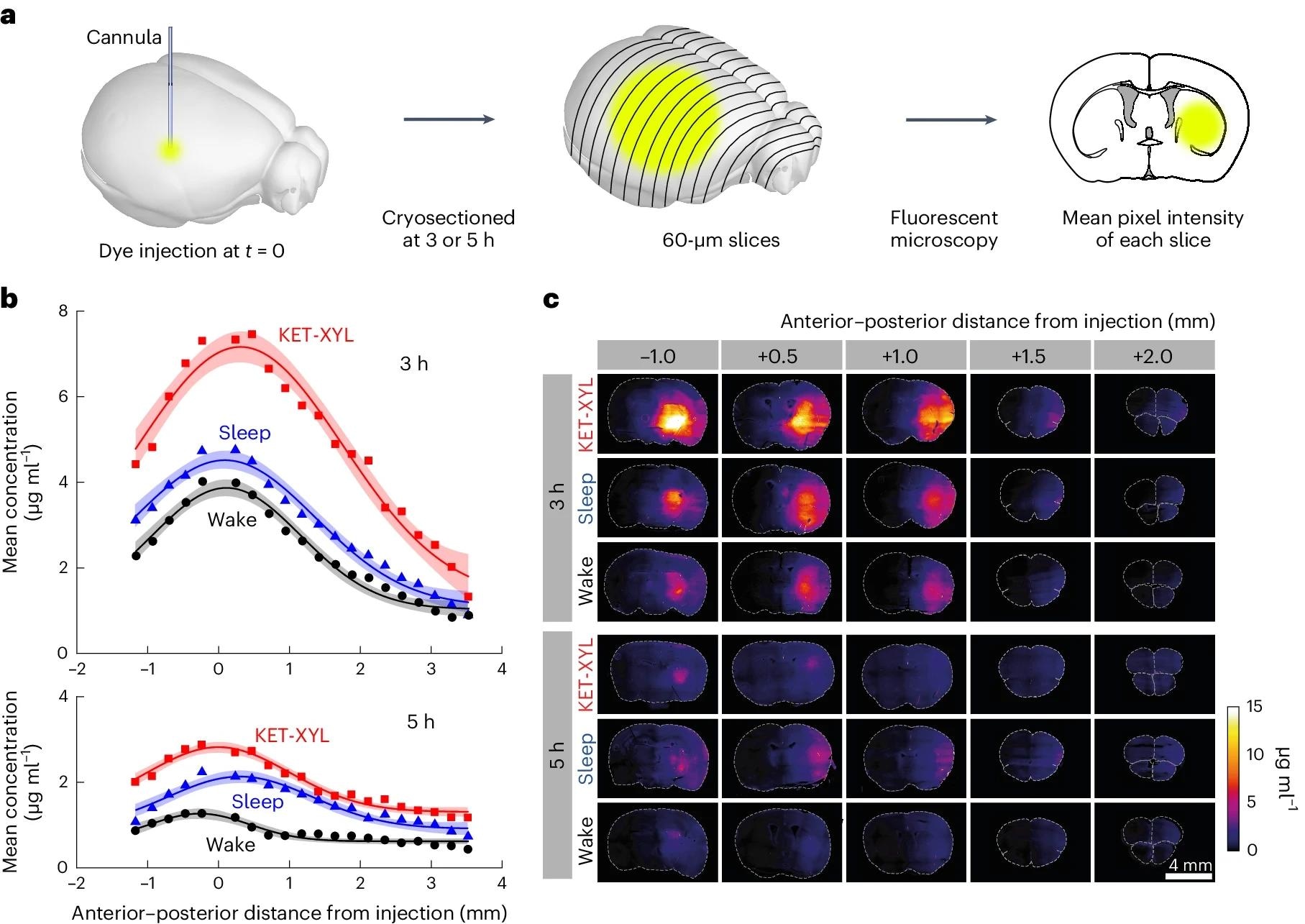 a, At either 3 or 5 h following injection of AF488 into the CPu, the brain was frozen and cryosectioned at 60 μm. The average fluorescent intensity across each slice was obtained by fluorescent microscopy; then the mean intensities across groups of four slices were averaged. b, The mean fluorescence intensity was converted to a concentration using the calibration data in Supplementary Fig. 1 plotted against the anterior–posterior distance from the point of injection for wake (black), sleep (blue) and KET-XYL (red) anesthesia. Top, the data after 3 h. Bottom, the data after 5 h. The lines are Gaussian fits to the data and the error envelopes show the 95% confidence intervals. At both 3 and 5 h, the concentrations during KET-XYL (P < 10−6 at 3 h; P < 10−6 at 5 h) and sleep (P = 0.0016 at 3 h; P < 10−4 at 5 h) were significantly larger than wake (two-way ANOVA with Bonferroni–Holm multiple comparisons correction). c, Representative images of the brain slices across the brain (anterior–posterior distance from the site of AF488 injection) at both 3 h (top three rows) and 5 h (bottom three rows). Each row represents data for the three vigilance states (wake, sleep and KET-XYL anesthesia).
a, At either 3 or 5 h following injection of AF488 into the CPu, the brain was frozen and cryosectioned at 60 μm. The average fluorescent intensity across each slice was obtained by fluorescent microscopy; then the mean intensities across groups of four slices were averaged. b, The mean fluorescence intensity was converted to a concentration using the calibration data in Supplementary Fig. 1 plotted against the anterior–posterior distance from the point of injection for wake (black), sleep (blue) and KET-XYL (red) anesthesia. Top, the data after 3 h. Bottom, the data after 5 h. The lines are Gaussian fits to the data and the error envelopes show the 95% confidence intervals. At both 3 and 5 h, the concentrations during KET-XYL (P < 10−6 at 3 h; P < 10−6 at 5 h) and sleep (P = 0.0016 at 3 h; P < 10−4 at 5 h) were significantly larger than wake (two-way ANOVA with Bonferroni–Holm multiple comparisons correction). c, Representative images of the brain slices across the brain (anterior–posterior distance from the site of AF488 injection) at both 3 h (top three rows) and 5 h (bottom three rows). Each row represents data for the three vigilance states (wake, sleep and KET-XYL anesthesia).
Of note, the diffusion kinetics were not different during anesthesia or sleep. Next, the team measured brain clearance during different vigilance states. They used a small volume of fluorescent dye, AF488, in mice injected with saline or anesthetic. This dye was reported to move freely in the parenchyma and could help quantify brain clearance accurately. Additionally, comparisons were made between wake and sleep states.
At the peak concentration, clearance was 70% to 80% in saline-injected mice, suggesting that standard clearance mechanisms were not disrupted. However, there was a substantial reduction in clearance with anesthetics (pentobarbital, dexmedetomidine, and ketamine-xylazine). Further, clearance was also reduced in sleeping mice relative to awake mice. Nevertheless, the diffusion coefficient was not significantly different between anesthesia and sleep states.
Besides, electroencephalogram (EEG) power spectra were measured; this indicated a weak correlation between delta power and peak clearance, suggesting lower clearance with deeper sleep. Histological experiments showed that the dye concentration three and five hours after injection was higher with ketamine-xylazine anesthesia and sleep. Data showed that AF488 redistribution was by diffusion alone and confirmed that both anesthesia and sleep inhibit clearance.
Conclusions
To conclude, the study illustrated reduced brain clearance during anesthesia and sleep, contradicting previous reports. Clearance might vary across anatomical locations, but the extent of variation might be small. Nevertheless, clearance inhibition by ketamine-xylazine was significantly independent of the location.
Nicholas P. Franks, one of the authors, said – "The field has been so focused on the clearance idea as one of the key reasons why we sleep that we were very surprised to observe the opposite in our results."
Notably, the findings are for a small volume of dye with free movement in the extracellular space. As such, larger molecules may exhibit different behavior. Besides, the exact mechanisms of how sleep and anesthesia impact brain clearance are unclear; however, the findings challenge the notion that the core function of sleep is brain toxin clearance.
Source:
Journal reference: