In a recent preclinical study published in the Journal of Clinical Investigation, researchers in the United States of America investigated the role of the transcription factor KLF15 (short for Kruppel-like factor 15) in maintaining white adipocyte properties in subcutaneous white adipose tissue (WAT) in mouse models and primary human adipose cells. They found that deleting Klf15 induces beige adipocyte properties in WAT and may affect systemic metabolism, thereby opening new avenues for treating obesity.
 Study: White adipocytes in subcutaneous fat depots require KLF15 for maintenance in preclinical models
Study: White adipocytes in subcutaneous fat depots require KLF15 for maintenance in preclinical models
Background
Adipocytes, key cells in mature adipose tissue, play roles in energy homeostasis and produce various paracrine and endocrine signals. Different adipose tissue depots have unique developmental and metabolic influences. Brown adipose tissue (BAT) and WAT differ significantly. While WAT matures after birth, BAT is present at birth, aiding the development of heat through β-adrenergic signaling, especially in response to cold. BAT's energy-burning capacity has the potential to treat obesity, but humans have limited BAT, which decreases with age.
Interestingly, subcutaneous WAT contains heterogeneous adipocytes, including white and 'beige' adipocytes, which share features with brown adipocytes. The origin of beige adipocytes remains unclear. Additionally, the factors maintaining white adipocyte properties are poorly understood. Understanding the depot-specific nature and context of these factors could reveal targets for obesity treatments.
KLF15 is a zinc finger transcription factor linked to lipid storage, adipogenesis, and BAT regulation. KLF dysregulation is reported to be associated with diseases like obesity and diabetes, highlighting the need for further research on KLFs' role in adipose tissue. Therefore, in the present preclinical study, researchers investigated the potential role of KL15 in maintaining white adipocyte properties, particularly in subcutaneous WAT depots.
About the study
In the present study, researchers examined Klf15 expression levels in three major types of adipose tissue: visceral, subcutaneous, and intracapsular brown, using wild-type mice. They also tested the impact of β-adrenergic stimulation on Klf15 expression. Additionally, they compared the expression levels of the three different adrenergic receptor family members (ADRB 1-3) across the adipose types and in human white and brown adipocytes. A Klf15-floxed mouse line was developed using CRISPR/Cas9 (short for Clustered regularly interspaced short palindromic repeats and associated protein 9) technology. Gene and protein expression analyses were conducted using qPCR (short for quantitative polymerase chain reaction), Western blot, and immunoblotting. Klf15-floxed mice were crossed with Adipoq-Cre mice to selectively delete Klf15 in mature adipocytes, generating Adipo-Klf15–cKO mice. Another mouse line, Prx1-Klf15 cKO, was generated by crossing Klf15-floxed mice with Prx1-Cre mice, targeting adipocyte progenitor cells in the iWAT depot. Functional assays included measuring oxygen consumption rates (OCR) and energy expenditure in response to adrenergic agonists using metabolic cages and Seahorse analyzers. Additionally, primary human subcutaneous adipocytes were used to test the conservation of KLF15 function, with Klf15 knockdown achieved through adenoviral infection.
Results and discussion
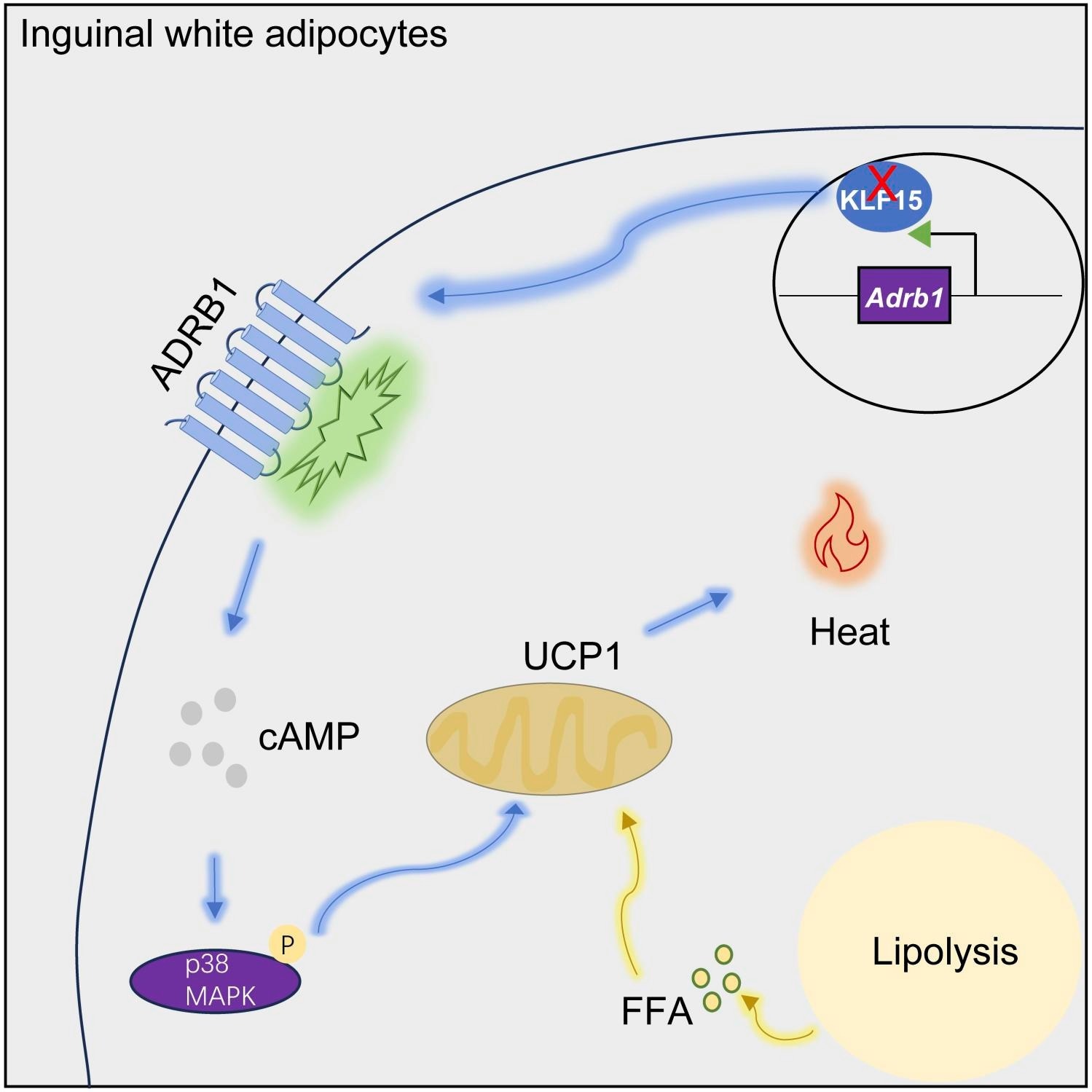
Klf15 expression was found to be approximately 75% lower in BAT compared to WAT, suggesting a physiological role for this difference. β-adrenergic stimulation in vivo resulted in the downregulation of Klf15 expression in WAT by about 50%. Among the adrenergic receptors, Adrb1 was the most differentially expressed in BAT compared to WAT, and a similar pattern was observed in human adipocytes. Overexpression of Adrb1 in white adipocytes was found to alter white adipocytes even in the presence of other adrenergic receptors.
Deletion of Klf15 in white adipocytes induced the expression of genes critical to brown fat identity and function, such as uncoupling protein 1 (Ucp1). This deletion also led to the upregulation of Adrb1, with other adrenergic receptors being unaffected or downregulated. The levels of β1AR increased with Klf15 deletion, suggesting that KLF15 modulates the maintenance of white adipocytes and "beiging" in subcutaneous WAT. In vivo, Adipo-Klf15–cKO mice exhibited a browner subcutaneous WAT with reduced mass and higher expression of brown fat genes, particularly in mature adipocytes isolated from the subcutaneous WAT, without changes in visceral WAT or BAT. The Prx1-Klf15 cKO mice showed reduced subcutaneous WAT mass, browner appearance, smaller adipocytes, and increased expression of brown fat marker genes. In vivo studies showed higher energy expenditure and better cold tolerance in Prx1-Klf15 cKO mice. Functional assays revealed that subcutaneous WAT with Klf15 deletion had enhanced OCRs in response to adrenergic stimulation, indicating increased adrenergic sensitivity. In human white adipocytes, the knockdown of KLF15 also resulted in increased Adrb1 and UCP1 expression, enhanced OCRs, and increased sensitivity to adrenergic stimulation.
Conclusion
In conclusion, these findings suggest that KLF15 modulates adipocyte sensitivity to β-adrenergic stimulation and is essential to maintaining white adipocyte properties in subcutaneous WAT. Targeting KLF15 could promote energy utilization through an alternative adrenergic pathway in white adipocytes. These discoveries enhance our understanding of adipose biology and the plasticity of mature white adipocytes. They also reveal previously unrecognized pathways that could potentially be more relevant and effective therapeutic targets against obesity in humans.
Journal reference:
- White adipocytes in subcutaneous fat depots require KLF15 for maintenance in preclinical models. Liang Li et al., Journal of Clinical Investigation,134(13):e172360 (2024), DOI: 10.1172/JCI172360, https://www.jci.org/articles/view/172360